Test: Infrared and Ultraviolet/Visible Spectroscopy - MCAT MCQ
10 Questions MCQ Test - Test: Infrared and Ultraviolet/Visible Spectroscopy
Which of the following statements accurately describes the molecular vibrations characteristic of IR spectroscopy?
Which of the following absorption peak or peaks would be most useful for identifying the compound diethylamine?
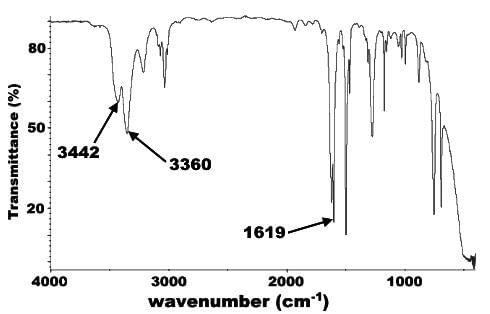
Which of the following statements about the IR spectrograph could indicate the successful completion of the reaction of pentanal with Tollen’s reagent?
What is the order of the following carbonyl compounds in decreasing wavenumber?
I. butanoyl chloride
II. ethyl butanoate
III. pentanal
IV. propanoic acid
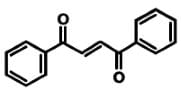
Based on the absorption spectrum, what is the likely name of the compound below?
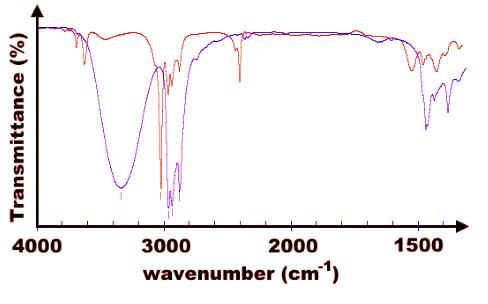
In the absorption spectrum provided below, assuming that the two plots represent the same compound, which of the following statements most accurately describes the graph?
Which of the following compounds can be characterized by UV-Vis spectroscopy?
Which of the following statements most accurately describes UV-Vis spectroscopy?
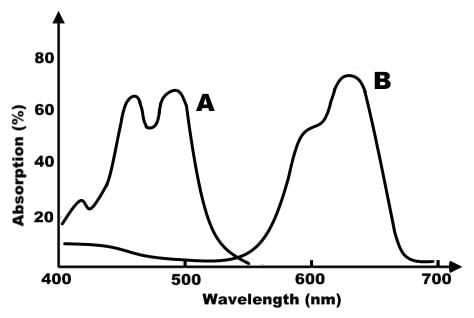
Based on the absorption spectrum below, which of the following plant pigments does the graph represent?
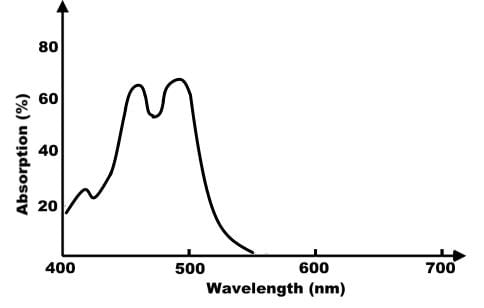
Based on the absorption spectrum below, which of the following statements about pigment B is true?