Test: Covalent Bonds - MCAT MCQ
10 Questions MCQ Test - Test: Covalent Bonds
Which of the following statements most accurately describes the details of the hybridization of sulfur hexafluoride?
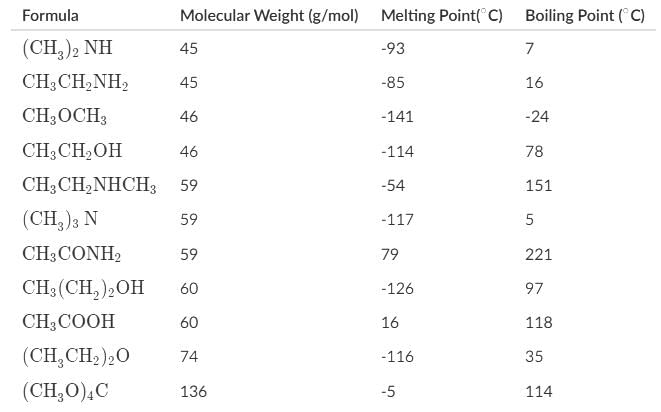
Which of the following statements about the melting and boiling points of the organic compounds can be deduced most accurately represent the data below?

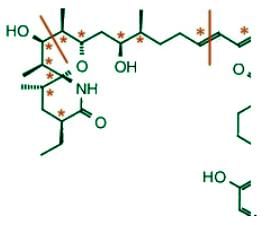
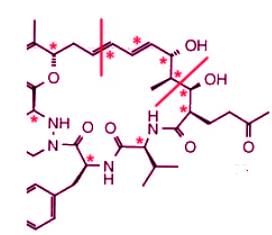
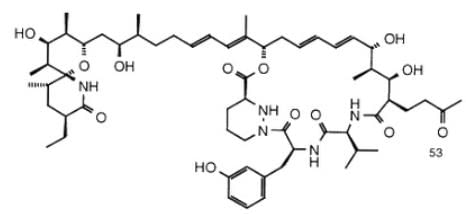
Sanglifehrin A (SFA) is a novel cyclophilin-binding compound showing immunosuppressive activity, but its role has not been entirely elucidated yet. How many stereogenic centers (R,S; E/Z) are there in the following compound sanglifehrin A?

Which of the following molecules does NOT have an atom that is sp-hybridized?
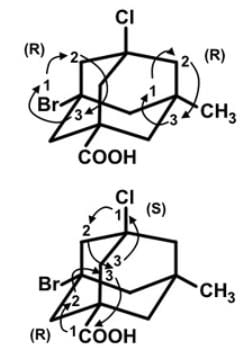
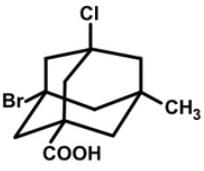
How many stereogenic centers are there in the following tetrasubstituted adamantane molecule?
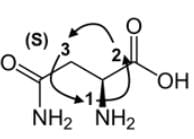
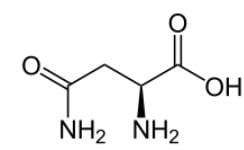
What is the systematic name of the following amino acid, which is commonly known as asparagine?
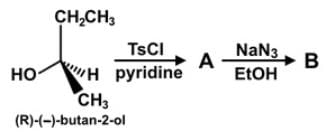
The parent compound, 2-butanol, undergoes a set of reactions to produce compounds A and B. Which of the following statements accurately describes the absolute and relative configurations of the compounds in the reaction series below?
Which of the following elements typically forms covalent bonds?
Which of the following pairs of atoms is likely to form a covalent bond?
Which of the following statements about covalent bonds is true?