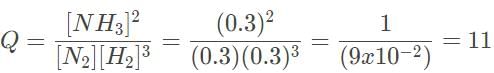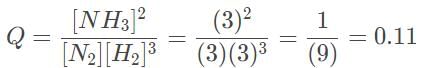Test: Chemical Equilibrium - MCAT MCQ
10 Questions MCQ Test - Test: Chemical Equilibrium
Which of the following is a true statement about the role of catalysts in a reaction?
I. Catalysts more effectively lowers the activation energy in the forward direction.
II. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product..
III. If a catalyst affects the equilibrium of the reaction, it must be consumed as the reaction proceeds.
IV. Catalysts can may increase the reaction rate or selectivity or enable the reaction at a lower temperature.
I. Catalysts more effectively lowers the activation energy in the forward direction.
II. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product..
III. If a catalyst affects the equilibrium of the reaction, it must be consumed as the reaction proceeds.
IV. Catalysts can may increase the reaction rate or selectivity or enable the reaction at a lower temperature.
Consider the following reaction:
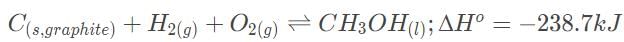
Which of the following would increase the value of Keq?
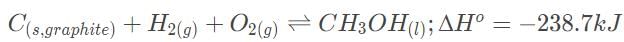
The Haber process involves the production of ammonia from hydrogen and nitrogen gas. In the laboratory, it was determined that the equilibrium concentrations of NH3, H2 and N2 are 0.0030 M, 0.10 M , and 0.090 M, respectively. Which of the following statements most accurately describes the reaction progress when all three concentrations are at 0.3 and 3.0 M?
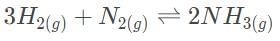
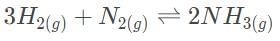
Which of the following is considered necessary as part of standard state conditions?
Which of the following statements most accurately describes the comparison between the rate constant k and the equilibrium constant keq?
What effect does increasing the temperature have on an exothermic reaction at equilibrium?
What happens to the value of the equilibrium constant (K) if the coefficients of a balanced chemical equation are multiplied by a factor?
Which of the following statements is true regarding a system at equilibrium?
What effect does increasing the pressure have on a system at equilibrium involving only gases?
How does decreasing the temperature affect an endothermic reaction at equilibrium?



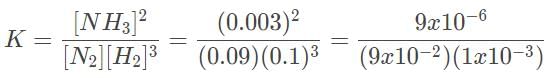 = 0.1
= 0.1