P. Bahadur Test: Redox Reactions & Electrochemistry - JEE MCQ
25 Questions MCQ Test - P. Bahadur Test: Redox Reactions & Electrochemistry
According to Faraday’s Second Law of Electrolysis the amounts of different substances liberated by the same quantity of electricity passing through the electrolytic solution are proportional:
Charge on one mole of electrons i.e. one Faraday is approximately:
A secondary cell after use ________ recharged by passing current through it in the ________ so that it can/can’t be used again.
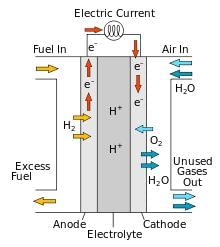
Fuel cells are _________ that are designed to convert the energy of combustion of fuels like hydrogen, methane, methanol, etc. directly into _________.
In corrosion, a metal is _________ of electrons to oxygen and results in the formation of oxides.
One of the simplest methods of preventing corrosion is to prevent the surface of the metallic object to come in contact with atmosphere. This can be done:
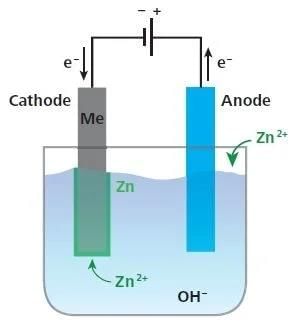
An electrochemical method is to provide a sacrificial electrode of another metal (like Mg, Zn, etc.) which corrodes itself but saves the object. A typical example is
Correct arrangement of Al, Cu, Fe, Mg and Zn in the order in which they displace each other from the solution of their salts is
The standard emf of galvanic cell involving 3 moles of electrons in its redox reaction is 0.59 V. The equilibrium constant for the reaction of the cell is
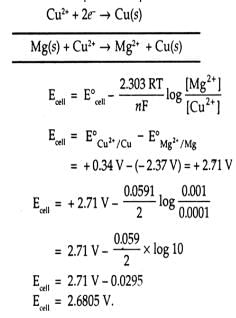
Emf of the cell
Mg (s) | Mg2+ (0.001M) || Cu2+ (0.0001M) | Cu(s) at 298 K is Cu Given : Eco2+, cu = +0.337 V, EºM22+ Me =- 2.37 V, F = 96500 C mol
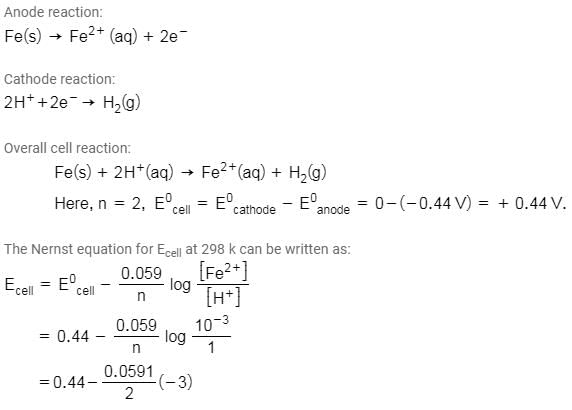
Emf of the cell
Fe (s) | Fe2+ (0.001 M)|| H+ (1M)|H2 (g) (1 bar) |Pt(s) at 298 K is
(Given: Eocell = +0.44 V)
The conductivity of 0.20 M solution of KCl at 298 K is 0.0248 S cm-1. Calculate its molar conductivity.
The resistance of a conductivity cell containing 0.001M KCl solution at 298 K is 1500 Ω. What is the cell constant if conductivity of 0.001M KCl solution at 298 K is 0.146 × 10-3 S cm-1.
How much charge is required for the reduction of 1 mol of Al3+ to Al?
How much electricity in terms of Faraday is required to produce 40.0 g of Al from molten Al2O3?
Three electrolytic cells A, B, C containing solutions of ZnSO4, AgNO3 and CuSO4, respectively are connected in series. A steady current of 1.5 amperes was passed through them until 1.45 g of silver deposited at the cathode of cell. How long did the current flow? What mass of copper and zinc were deposited?
The standard emf of galvanic cell involving 3 moles of electrons in its redox reaction is 0.59 V. The equilibrium constant for the reaction of the cell is
An increase in equivalent conductance of a strong electrolyte with dilution is mainly due to
For the reduction of silver ions with copper metal the standard cell potential was found to be +0.46V at 25°C. The value of standard Gibbs energy, ΔG° wll be (F = 96500 C mol-1)
Which of the following electrolytic solutions has the least specific conductance?
The highest electrical conductivity of the following aqueous solutions is of
The standard electrode potential is measured by
When KMnO4 acts as an oxidizing agent and ultimately forms MnO42-, MnO2, Mn2O3, and Mn2+ then the number of electrons transferred in each case
On the basis of the following E° values, the strongest oxidizing agent is: