HOTS Questions: Atoms and Molecules - Delhi Police Constable MCQ
25 Questions MCQ Test - HOTS Questions: Atoms and Molecules
Who suggested that if we go on dividing matter, a stage will come when particles obtained can’t be divided further?
What information we get from molecular formula
A It represents one molecule of the substance
B It does not tells the name of the substance
C It tells about the type of atoms
D It represents formula mass unit of one substance
A It represents one molecule of the substance
B It does not tells the name of the substance
C It tells about the type of atoms
D It represents formula mass unit of one substance
Calculate the number of Mg atoms in 0.024 g of Mg
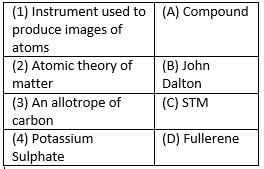
Who was the first to use the symbols for elements?
What according to Dalton’s atomic theory is true among the following-
A. Atom is divisible into Protons, electrons and neutrons
B. Atoms of the same element have different atomic masses.
C. Atoms are the ultimate indivisible particle of matter
D. Atoms of same element have same atomic masses size and chemical properties
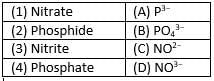
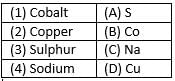
Which of the following elements are present in Quick lime?
Statement A: mole is quite often known as chemists dozen
Statement B: the mass of one twelfth (1/12) of the mass of one atom of carbon taken as 1u.
Which of the two statement is true
During a chemical reaction, the sum of the masses of the reactants and products remains unchanged. This is known as
How many molecules are present in one gram molecular mass of a substance?
Statement A: Atoms can exist independently
Statement B: The law of constant proportions is applicable only to pure chemical compounds.
Which of the two Statements is true?
What is the mass of 0.5 mole of Hydrogen atom
The atomic mass of calcium is 40 the number of moles in 60 g of calcium are
What is the name given to the short hand representation of an element?
Which postulates of Dalton’s atomic theory is the basis of the law of conservation of mass?



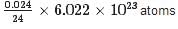 = 6.022 x 1020 atoms.
= 6.022 x 1020 atoms.