Important Questions (1 mark): Structure of the Atom - Delhi Police Constable MCQ
25 Questions MCQ Test - Important Questions (1 mark): Structure of the Atom
Which of the following is regarded as a universal particle?
The main subatomic particles of nucleus are-
What is name given to the number of protons in the nucleus of the atom?
The atom which does not contain any neutron in the nucleus is
Match the following with correct response.

There are 14 protons and 13 neutrons on the nucleus of an element. What is its mass number?
“Electrons present in the extra nuclear portion are not stationary rather they revolve around the nucleus at high speed following a circular path” Who said this?
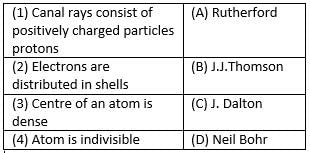
Match the following with correct response.
Nitrogen atom has atomic mass of 14 u and has 7 protons in its nucleus. How many neutrons does it have?
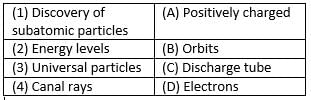
Match the following with correct response.

In an alpha scattering experiment, few alpha particles rebounded because
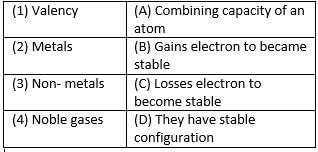
Match the following with correct response.
Isotopes of an element have
A. Same chemical properties
B. Same atomic masses
C. Differeat atomic numbers
D. Atoms of same element
How many times is radius of extra nuclear portion more than that of the nucleus of an atom?
Match the following with correct response.
An atom has a mass number of 23 and atomic number 11. The number of protons are_________.
How many electrons are present in the species He2+ion?
Who got the Noble Prize for the work on the structure of atom?
Which of the following is not observed by Rutherford in the α-particle scattering experiment?
A. Most of the α-particle rebound after hitting the gold foil
B. Some of the particles deflect by their path
C. Some of the particles did not pass through the gold foil
D. Most of the particles pass straight through the gold foil
In a species, the number of electrons is more than the number of protons. Predict its nature.
Out of L and M shells which is near to the nucleus of an atom?
Which of the following is correct about atom?
A. It is the smallest unit of matter
B. Size of atom is very large as compared to the nucleus of the atom
C. In an atom number of protons is equal to number of electrons
D. Atoms are stable



















