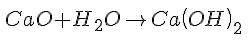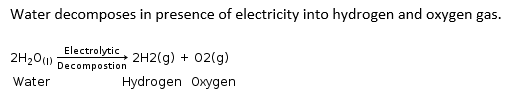Test: Combination And Decomposition Reaction - Class 10 MCQ
15 Questions MCQ Test - Test: Combination And Decomposition Reaction
Which of the following product is formed when calcium oxide reacts with water?
We use a solution of slaked lime to whitewash the walls. In the beginning the wall looks rough and coarse, but after 2 – 3 days the wall appears smooth and shiny. This is due to the formation of:
The reaction of calcium oxide with water to form calcium hydroxide is an example of:
Combination reactions can take place between:
CaO + H2O → Ca(OH)2 is an example of a:
An example of combination reaction between an element and compound is:
Which of the following reaction requires light?
Which of the following is a combination reaction?
Which of the following decomposition reaction occurs with the absorption of electrical energy?
When a magnesium ribbon is burnt in air, the ash formed is
Which of the following does denote chemical reaction take place?
Which of the following product is formed after heating of limestone?
Which of the following is a use of an electrolytic decomposition reaction?
A student uses lime water to test the gas evolved as a result of action of dilute HCl on solid sodium carbonate. The chemical compound present in lime water is :