Test: Types Of Displacement Reactions - Class 10 MCQ
10 Questions MCQ Test - Test: Types Of Displacement Reactions
Which of the following is an example of displacement reaction?
Identify the reaction:
AgNO3(aq) + KCl(aq) → AgCl↓ + KNO3(aq)
The reaction, Zn + 2HCl → ZnCl2 + H2 is an example of:
The reaction between lead nitrate and potassium iodide is an example of _______ .
Fe2O3 + 2Al → Al2O3 + 2Fe
The above reaction is an example of a:
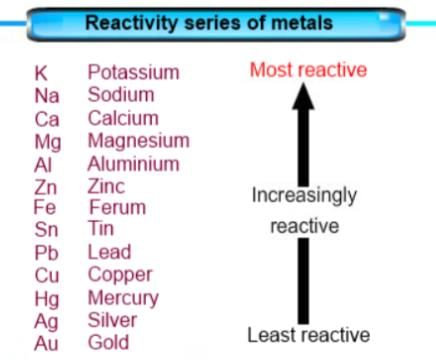
Which of the following metals comes above zinc in reactivity series?
The given reaction indicates that:
Cu + 2AgNO3 → Cu(NO3)2 + 2Ag
Which form of energy is responsible for the decomposition reactions ?
Why does an iron nail become brownish in color and the blue color of copper sulfate solution fades?
Assertion (A): The iron nail becomes brownish in color and the blue color of copper sulfate solution fades.
Reason (R): In the reaction Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s), iron displaces copper in the copper sulfate solution.