Test: Alkenes Electrophilic Addition Reaction - JEE MCQ
21 Questions MCQ Test - Test: Alkenes Electrophilic Addition Reaction
Direction (Q. Nos. 1 - 7) This section contains 7 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. What is the major addition product in the following reactions?

What is the major product in the following reaction?
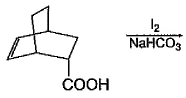
Consider the following reaction.
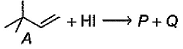
Molecular formula of both P and Q are C6H13l
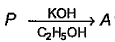 tetra substituted alkene isom er of A. Hence, P is most likely
tetra substituted alkene isom er of A. Hence, P is most likely
If trans-2-pentene is treated with Br2 in CCI4 in presence of Lewis acid FeBr3
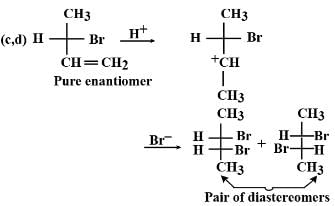
3-methyl-1-pentene has a chiral carbon. If a pure enantiomer of 3-methyl-1-pentene is treated with HBr, the correct statement regarding major bromoalkane formed is
Consider the following reaction.
Which of the following would not be formed in the above reaction?
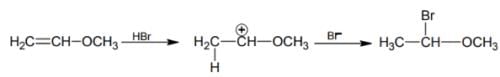
HBr reacts with CH2 = CH – OCH3 under anhydrous conditions at room temperature to give
[AlEEE 2006]
Direction (Q. Nos. 8 - 13) This section contains 6 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. In which of the following reaction(s), major product is formed by the rearrangement of reactive intermediate?
Consider the following reaction.
The correct statement(s) is/are
In which of the following reactions, reactants and products are correctly matched ?
Which is the important reactive in termediate in the following reaction ?
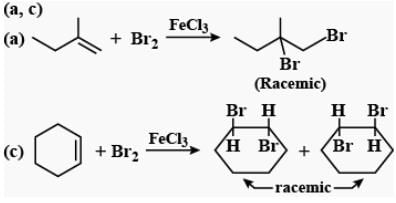
In which of the following halogen addition reactions, racemic mixture of products is formed?
Consider the following addition reaction on a pure enantiomer of the shown bromoalkene.
Q. What is/are true regarding products of the above reaction ?
Direction (Q. Nos. 14 - 16) This section contains a paragraph, describing theory, experiments, data, etc.
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
A mixture of hydrocarbons contain three compounds P, Q and R. Q and R are stereoisomers. When this mixture is treated with cold, concentrated H2SO4 , Q and R got a bsorbed while Premained unreacted , forming a separate layer at surface. After separation of P, the acid solution is heated and the hydrocarbons Q and R recovered, containing predominantly Q. When vaporised mixture of P, Q and R is passed over heated Ni containing adsorbed H2 gas, a single alkane of six carbon was obtained. Also, Q on treatment with Br2/CCI4 in the presence of FeBr3, a meso dibromide is formed.
Q. What is P ?
A mixture of hydrocarbons contain three compounds P, Q and R. Q and R are stereoisomers. When this mixture is treated with cold, concentrated H2SO4 , Q and R got a bsorbed while Premained unreacted , forming a separate layer at surface. After separation of P, the acid solution is heated and the hydrocarbons Q and R recovered, containing predominantly Q. When vaporised mixture of P, Q and R is passed over heated Ni containing adsorbed H2 gas, a single alkane of six carbon was obtained. Also, Q on treatment with Br2/CCI4 in the presence of FeBr3, a meso dibromide is formed.
Q. Q and R respectively are
A mixture of hydrocarbons contain three compounds P, Q and R. Q and R are stereoisomers. When this mixture is treated with cold, concentrated H2SO4 , Q and R got a bsorbed while Premained unreacted , forming a separate layer at surface. After separation of P, the acid solution is heated and the hydrocarbons Q and R recovered, containing predominantly Q. When vaporised mixture of P, Q and R is passed over heated Ni containing adsorbed H2 gas, a single alkane of six carbon was obtained. Also, Q on treatment with Br2/CCI4 in the presence of FeBr3, a meso dibromide is formed.
Q. If Q and R are treated separately with HBr, the correct statement concerning addition product is
Direction (Q. No. 17 - 20) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q. If a pure enantiomer of 5 -bromo -2 -hexene is treated with Br2— CCI4 , how many different tribromides would be formed ?
If a racemic mixture of 3 -methyl- 1-pentene is treated with HCl, how many different chloropentane (importantproducts only) would be formed?
Consider the following reaction.
Q. How many different products would be formed?
Consider the following reaction and the major product.
Q. If energy profile of the above reaction is drawn, how many transition states would be observed?
Direction (Q. No. 21) Choices for the correct combination of elements from Column I and Column lI are given as options (a), (b), (c) and (d), out of which one is correct.
Consider the reactions in Column I and match with the properties of products from Column II.