Test: Alkynes Preparation & General Properties - NEET MCQ
16 Questions MCQ Test - Test: Alkynes Preparation & General Properties
Direction (Q. Nos. 1 - 8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
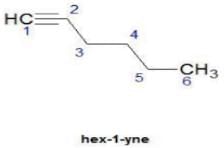
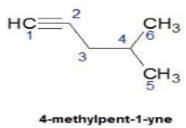
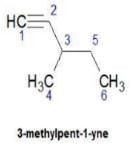
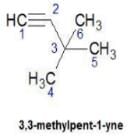
Q. How many distinct alkynes exist for C6H10 which gives effervescence on heating with Na?
Which of the following improperly describes the physical property of an alkyne?
What two atomic or hybrid orbitals overlap to form the carbon-carbon σ (sigma) bond in ethyne?
Which of the following statements correctly describes the general reactivity of alkynes?
How many different isomeric alkynes on catalytic hydrogenation gives the same 3-ethyl hexane?
An organic compound X (C6H13Br) is optically active. X on treatment with (CH3)3COK in (CH3)3COH gives Y (C6H12), a major product. Y on treatment with Br2— CCI4 in the presence of FeBr3 gives a dibromide which on further treatment with NaNH2 gives C6H10 which is still optically active. Hence, X and Y respectively are
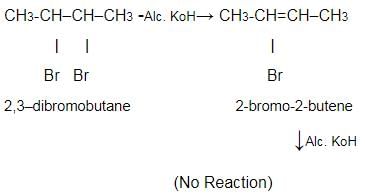
When 2,3-dibromobutane is treated with KOH in ethanol, 2-bromo-2-butene is formed which does not undergo further dehydrobromination to form 2-butyne under similar condition because
When vicinal dibromide is heated with KOH in ethanol (~ 200°C), double dehydrohalogenation takes place giving alkyne. Which of the following fails to give alkyne according to this procedure?
Direction (Q. Nos. 9 -12) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. In which of the following reaction(s), an alkyne product is formed?
Which of the following reagents gives an effervescence when reacted with 1-butyne?
Which of the following reagent(s) can be used to distinguish between 1-hexyne and 2-hexyne?
Terminal alkyne being therm odynamically less stable than an internal alkyne, instead when 2,2-dibromo butane is treated with NaNH2,1-butyne is formed as major product, not 2-butyne because
Direction (Q. Nos. 13 - 16) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement I : When 1-butyne is treated with NaNH2, sodium salt is formed.
Statement II : NH3 is w eaker acid than .
Statement I : Treatment of either 1,2-dibromobutane or 2,2-dibromobutane with NaNH2 gives the same 1-butyne.
Statement II : NaNH2 forms salt with terminal alkyne which makes possible the above observation.
Statement I : Propyl lithium on reaction with 1-bromo-1-pentyne gives 4-octyne.
Statement II : Propyl lithium acts as a very strong nucleophile, brings about SN2 reaction of halides.
Statement l : C6H5MgBr with propyne gives 1-phenyl propyne.