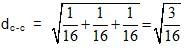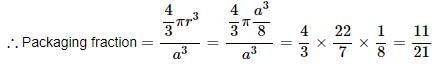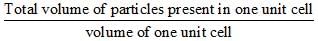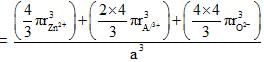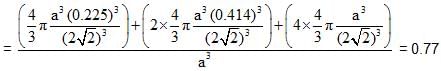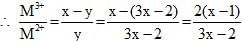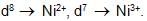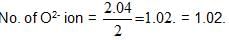P. Bahadur Test: The Solid State (Old NCERT) - JEE MCQ
15 Questions MCQ Test - P. Bahadur Test: The Solid State (Old NCERT)
Acrystal is made of particles A and B. A forms FCC packing and B occupies all the octahedral voids. If all the particles along the plane as shown in figure are removed, then, the formula of the crystal would be:
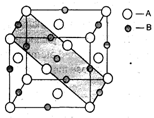
A crystal is made of particle X, Y & Z. X forms FCC packing, Y occupies all octahedral voids of X and Z occupies all tetrahedral voids of X, if all the particles along one body diagonal are removed then the formula of the crystal would be –
In a hypothetical solid C atoms are found to form cubical close packed lattice. A atoms occupy all tetrahedral vioids & B atoms occupy all octahedral voids. A and B atoms are of appropriate size, so that there is no distortion in CCP lattice of C atoms. Now if a plane as shown in the following figure is cut.Then the cross section of this plane will look like.
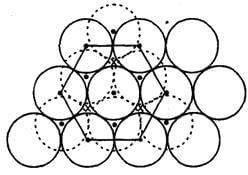
In hexagonal close packing of sphere in three dimensions.
Diamond has face-centred cubic lattice. There are two atoms at (0,0,0) and coordinates.The ratio of the carbon-carbon bond distance to the edge of the unit cell is
The following diagram shows arrangement of lattice point with a = b = c and α = β = γ = 900.
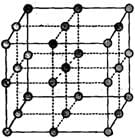
Choose the correct options.
Consider a cube containing n unit cells of a cubic system. A plane ABCD obtained by joining the mid points of the edges on one of its identical faces had atoms arranged as shown. Let p be the packing fraction. Choose the correct option:
Spinel is an important class of oxides consisting of two types of metal ions with the oxide ions arranged in CCP pattern. The normal spinel has 1/ 8 of the tetrahedral holes occupied by one type of metal ion and 1/2 of the octahedral holes occupied by another type of metal ion. Such a spinel is formed by Zn2+, A13x and 02- with Zn2+ in the tetrahedral holes. If CCP arrangement of oxide ions remains undistorted in the presence of all the cations, formula of spinel and fraction of the packing fraction of crystal are respectively :
What is the maximum number of layers of atoms in close packed planes that will lie within two imaginary parallel planes having a distance between them of R (where R is the radius of atom) in the copper crystal (FCC) ? Consider the atoms to be within the parallel planes if their centres are on or within the two parallel planes.
A transition Metal M can exist in two oxidation states +2 and +3.It forms an oxide whose experimental formula is given by MxO where x < 1. Then the ratio of metal ions in +3 state to those in +2 state in oxide is given by :
Analysis show that nickel oxide consist of nickel ion with 96% ions having d8 configuration and 4% having d7 configuration. Which amongst the following best represents the formula of the oxide.
Which of the following statements is correct about the conduction of electricity in pure crystal of silicon at room temperature?
Statement-1 : An important feature of Fluorite structures is that cations being large in size occupy FCC lattice points whereas anions occupy all the tetrahedral voids giving the formula unit AB2 (A : cation, B : anion).
Statement-2 : There are 6 cations and 12 anions per FCC unit cell of the Fluorite structure.
Statement-1 : In NaCI crystal each Na ion is touching 6 CI– ions but these CI- ions do not touch each other.
Statement--2 : The radius ratio roe rNa+/rCI- is greater than 0.414, required for exact fitting