Test: Tests & Distinctions - NEET MCQ
20 Questions MCQ Test - Test: Tests & Distinctions
Only One Option Correct Type
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Tollen’s reagent used for the distinction of aldehydes with ketones is
Which compound given below does not form red precipitate with ammoniacal solution of Cu(lI) tartarate ?
Which reagent can differentiate between benzaldehyde and acetophenone?
Reagent that can differentiate 2-propanoi from acetone is
Which reagent given below can differentiate propanal from propanone?
Which of the following would produce an orange coloured precipitate with 2, 4-dinitrophenyl hydrazine?
One or More than One Options Correct Type
Direction (Q. Nos. 9-14) This section contains 6 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. Which of the following form(s) yellow precipitate with NaOH/I2 solution?
Which of the following compounds reduce(s) ammoniacal silver nitrate solution?
Which of the following fail(s) to reduce Fehling’s solution?
Which of the following reagents can be used for the separation of a mixture containing CH3CHO and CH3CN?
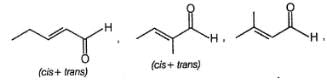
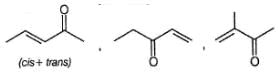
One mole of a symmetrical alkane on ozonolysis gives two moles of an aldehyde having molecular mass of 44u. The alkene is:
Which compound(s) given below, upon acid hydrolysis gives a compound that forms a yellow precipitate with KOH/I2 solution?
Comprehension Type
Direction (Q. Nos. 15-17) This section contains a paragraph, describing theory, experiments, data, etc.
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
A neutral organic compound A(C10H20O2) neither reduces Tollen’s reagent nor forms precipitate with 2, 4-dinitrophenyl hydrazine, but can be resolved into enantiomers. A on acid hydrolysis forms two compounds B and C, both are enantiomeric. C neither reduces Fehling’s solution nor forms iodoform with alkaline iodine solution. C on oxidation with CrO3/HCI /pyridineforms D which is still resolvable into enantiomers. D on further treatment with aqueous (C2H5O)3 Al solution gives back A.
Q.
How many different stereoisomers exist for A ?
A neutral organic compound A(C10H20O2) neither reduces Tollen’s reagent nor forms precipitate with 2, 4-dinitrophenyl hydrazine, but can be resolved into enantiomers. A on acid hydrolysis forms two compounds B and C, both are enantiomeric. C neither reduces Fehling’s solution nor forms iodoform with alkaline iodine solution. C on oxidation with CrO3/HCI /pyridineforms D which is still resolvable into enantiomers. D on further treatment with aqueous (C2H5O)3 Al solution gives back A.
Q.
What ist rue regarding D ?
A neutral organic compound A(C10H20O2) neither reduces Tollen’s reagent nor forms precipitate with 2, 4-dinitrophenyl hydrazine, but can be resolved into enantiomers. A on acid hydrolysis forms two compounds B and C, both are enantiomeric. C neither reduces Fehling’s solution nor forms iodoform with alkaline iodine solution. C on oxidation with CrO3/HCI /pyridineforms D which is still resolvable into enantiomers. D on further treatment with aqueous (C2H5O)3 Al solution gives back A.
Q.
The correct statement regarding the following reaction is
CH3CHO and C6H5CH2CHO can be distinguished chemically by:
How many different isomers of C5H8O, on treatment with 2, 4-dinitrophenyl hydrazine gives orange precipitate?
One mole of an organic compound 'A' with the formula C3H8O reacts completely with two moles of HI to form X and Y. When 'Y' is boiled with aqueous alkali it forms Z. Z answers the iodoform test. The compound 'A' is ______.
Matching List Type
Direction (Q. No. 21) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
Q.
Match the qualitative tests listed in Column I with compounds from Column II that gives positive response to these tests and mark the correct option from the codes given below.