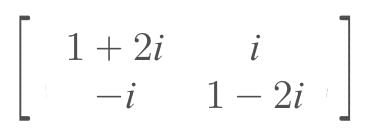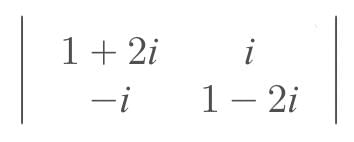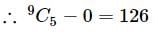KIITEE Mock Test - 7 - JEE MCQ
30 Questions MCQ Test - KIITEE Mock Test - 7
A mirror produces magnified erect image of an object. The nature of the mirror is
If the speed of an electron of hydrogen atom in the ground state is 2.2 x 106 m/s, then its speed in the third excited state will be
Two pendulums begin to swing simultaneously. If the ratio of the frequency of oscillations of the two is 7 : 8, then the ratio of lengths of the two pendulums will be
The vectors (A + B) and (A - B) are at right angles to each other. This Is possible under the condition
In the study of transistor as an amplifier, the ratio of collector current to emitter current is 0.98 then the ratio of collector current to base current will be
The angular momentum of the Earth revolving around the Sun is proportional to Rn, where R is the distance between the Earth and the Sun. The value of n is
The absolute enthalpy of neutralisation of the reaction MgO(s) + 2HCl(aq) → MgCl2(aq) + H2O(l) will be
Out of Cu, Ag, Fe and Zn, the metal which can displace all others from their salt solutions is
The formula mass of Mohr's salt is 392. The iron present in it is oxidised by KMnO4 in acidic medium. The equivalent mass of Mohr's salt is
Which of the following unit cell dimensions are correct for tetragonal crystal system?
Based on first Jaw of thermodynamics which of the following is correct
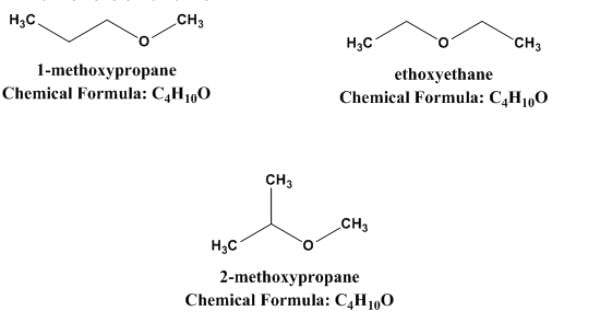
How the molecular formula C4H10O represents many metameric ethers?
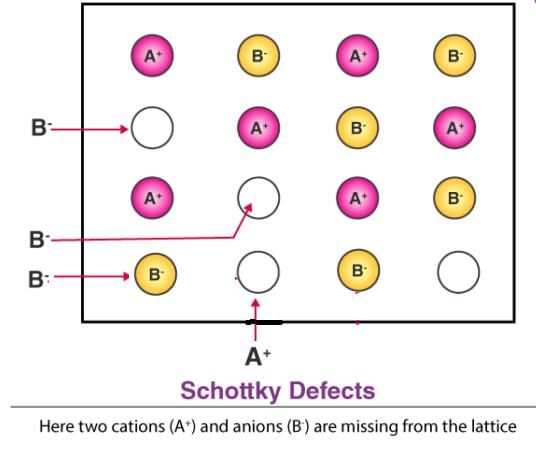
Which among the following statements is true about the Schottky defect?
The products obtained when anisole is heated in a sealed tube with HI are
How many gram of sodium (atomic mass 23 u) is required to prepare one mole of ethane from methyl chloride by Wurtz reaction?
p and q are the roots of x2 + ax + b = 0, then equation whose roots are p2q, q2p is
If the sum of the slopes of the lines given by x² – 4p x y + 8y² = 0 is three times their product, then p =
If the roots of 5x2 + 13x + k = 0 are reciprocal of each other, then the value of k is____
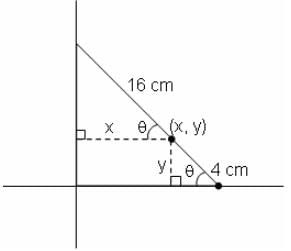
A bar of length 20 cm moves along with its extremities on two fixed straight lines (taken as axes) at right angles. If a marked point on it is at 4 cm from one end, then the eccentricity of the ellipse described by the marked point is
A man is known to speak truth 3 out of 4 times. He throws a die and reports that it is a six. The probability that it is actually a six is
Let S={1,2,3,….9}. For k=1,2,…5, let Nk be the number of subsets of S, each containing five elements out of which exactly k are odd. Then N1 + N2 + N3 + N4 + N5 =


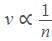

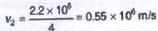

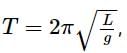 now if the acceleration due to gravity is g.
now if the acceleration due to gravity is g.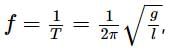
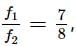 it means,
it means,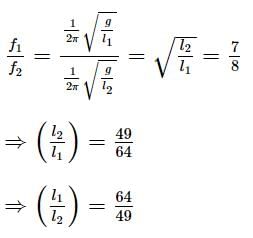


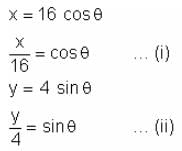
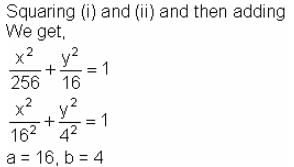
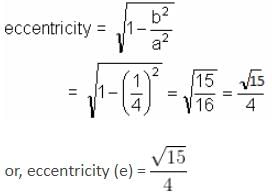
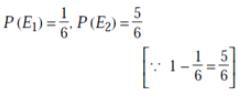
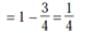
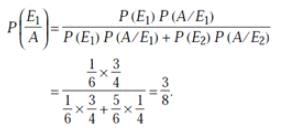
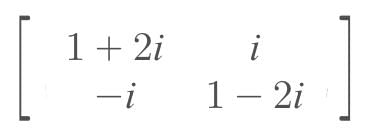 where i = √-1, then A(adj A) = …..
where i = √-1, then A(adj A) = …..