Test: Atoms - JEE MCQ
30 Questions MCQ Test - Test: Atoms
According to ‘plum pudding model’ atoms on the whole are electrically neutral because
What is the shortest wavelength present in the Paschen series of spectral lines?
The average angle of deflection of α-particles by a thin gold foil as predicted by Thomson’s model is
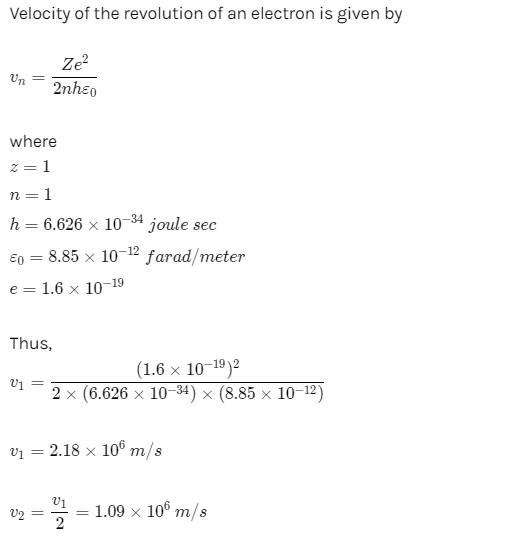
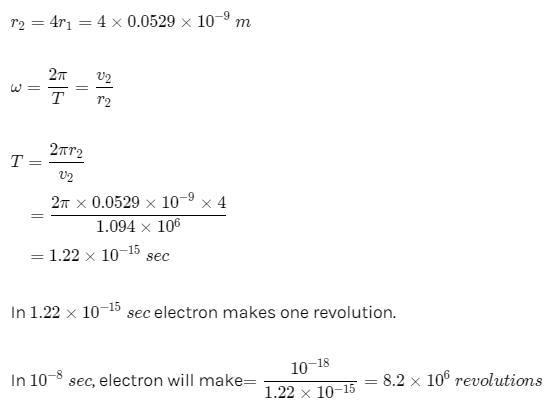
The average lifetime of the first excited level of a hydrogen atom is 1.0 ×10−8 s. In the Bohr model, how many orbits does an electron in the n = 2 level complete before returning to the ground level?
Which of these statements about Bohr model applied to hydrogen atom correct?
Probability of backward scattering (i.e., scattering of α -particles at angles greater than 90∘) predicted by Thomson’s model is
In Geiger-Marsden experiment very small deflection of the beam was expected because
Suppose you are given a chance to repeat the alpha-particle scattering experiment using a thin sheet of solid hydrogen in place of the gold foil. (Hydrogen is a solid at temperatures below 14 K.) What results do you expect?
It is found experimentally that for small thickness t, the number of α-particles scattered at moderate angles is proportional to t. What clue does this linear dependence on t provide?
An electron collides with a hydrogen atom in its ground state and excites it to a state of n = 3. How much energy was given to the hydrogen atom in this inelastic collision?
Which of these statements correctly describe the atomic model according to classical electromagnetic theory ?
In the ground state of which model electrons are in stable equilibrium with zero net force?
A triply ionized beryllium ion Be3+, (a beryllium atom with three electrons removed), behaves very much like a hydrogen atom except that the nuclear charge is four times as great. For the hydrogen atom, the wavelength of the photon emitted in the n =2 to n=1 to transition is 122 nm. What is the wavelength of the photon emitted when a Be3+ ion undergoes this transition?
Fluorescent lamps are more efficient than incandescent lamps in converting electrical energy to visible light because
In which of the models An atom has a nearly continuous mass distribution?
Find the longest wavelength present in the Balmer series of hydrogen, corresponding to the H- line.
The model that best explains the results of Geiger-Marsden experiment is
In a Geiger -Marsden experiment, what is the distance of closest approach d to the nucleus of a 7.7 MeV α−particle before it comes momentarily to rest and reverses its direction?