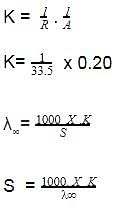KVS PGT Chemistry Mock Test - 4 - KVS PGT/TGT/PRT MCQ
30 Questions MCQ Test - KVS PGT Chemistry Mock Test - 4
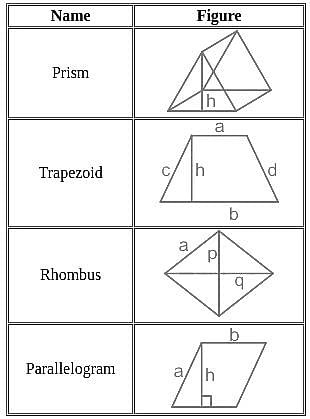
Directions: Improve the bracketed part of the sentence.
Q. He (like) to picture himself as an original thinker.
In the following question, out of the four alternatives, select the alternative which is the best substitute for the phrase.In the following question, out of the four alternatives, select the alternative which is the best substitute for the phrase.
Q. A man who is courteous and gallant in his behavior.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Improve the bracketed part of the sentence with the parts given below.
Q. India (will soon hold) talks on the issue of militancy with Afghanistan.
Directions: In the following question, one part of the sentence may have error(s). Find out the part of the sentence having an error and select the appropriate option. If a sentence is free from error, select 'No error' as your answer.
Q. You have been doing (1)/ your project work (2)/ routinely? (3)/ No error (4)
Choose the alternative which is an odd word/number/letter pair out of the given alternatives.
How to delete a particular word in a document using shortcut key?
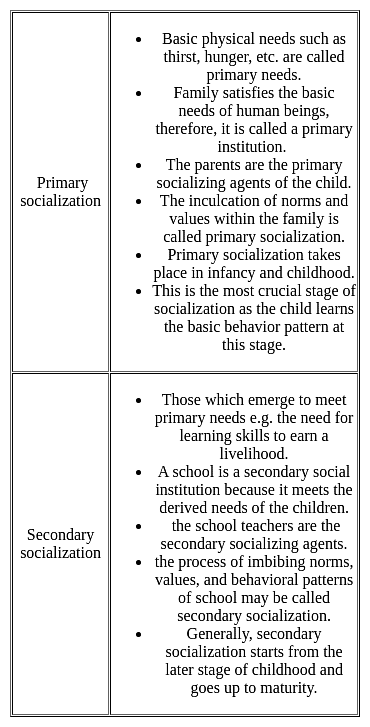
In the evening, Kalpesh went to a nearby park to observe young/small children playing (2- 6 years of age) usually escorted by their parents. He noticed the interacting patterns of parents and their children in the park. Two situations were observed by Kalpesh.
Situation 1: A couple scolded the child when she was asking for the ice cream.
Situation 2: A couple was playing hide and seek with their child.
These two situations are examples of which type of socialization
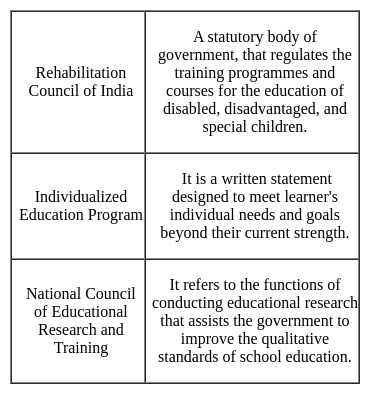
This type of counselling involves people who drop out from the school and whose difficulties are likely to be more severe.
The catalytic activity of transition metals and their compounds is mainly due to
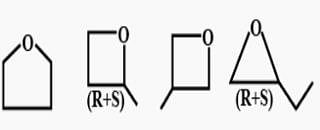
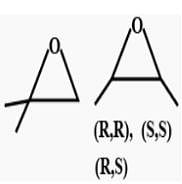
Mono-chloro product (inculding steroisomers)are :
The nature of bonds in the compounds of carbon is mostly:
How many structural isomers exist for C4 H80 which are simultaneously ether? Also there is no atom sp2-hybridised.
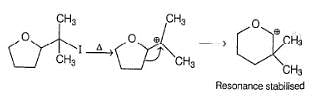
Based on the following reaction,
It can be concluded that
Which of the following carbohydrates is called milk sugar?
Which of the following correctly represents H-bonding?
The half life of a reaction is halved as the initial concentration of the reactant is doubled. The order of the reaction is
One of the electrons of the highest energy level is taken to next excited state in the following diatomic species. Select the species which undergoes change in bond order?
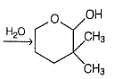
Which is the most likely product when the following iodide is heated with water?
The solubility of [Co(NH3)4Cl2] CIO4_________ if the = 50,
= 70, and the measured resistance was 33.5Ω in a cell with cell constant of 0.20 is ____.
Which of the following gaseous molecule is monoatomic?
Out of the following ions, which pair wil make the compound most covalent?