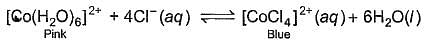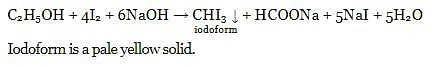KVS PGT Chemistry Mock Test - 7 - KVS PGT/TGT/PRT MCQ
30 Questions MCQ Test - KVS PGT Chemistry Mock Test - 7
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The Right to Education (RTE) Act was enacted by the Parliament of India in the year _____.
Which of the following are the examples of strong nucleophiles but weak base in protic solvents?
I. CH3S-
II. CH3O-
III. I-
IV. H2O
V. F-
Mole fraction of ethanol in ethanol-water solution is 0.25. Thus, this solution is
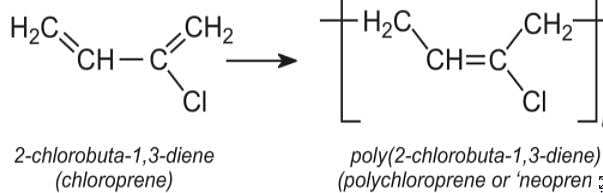
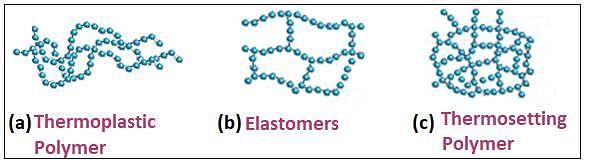
A synthetic polymer which resembles natural rubber is:
What does hydrogen peroxide liberate from potassium iodide?
The weakest inter-particle forces are present in:
When hydrochloric acid is added to cobalt (II) nitrate solution at room temperature, the following reaction takes place
Q. The solution is blue at room temperature. However, it turns pink when cooled in a freezing mixture. Based upon this information, which of the following expression is correct for the forward reaction?
18 g of glucose (C6H12O6) is added to 178.2 g of water. The vapour pressure of water for this aqueous solution at 100º C is -
[AIEEE 2006]
Which of the following will have three stereoisomeric form ?
i. [Cr(NO3)3(NH3)3]
ii. K3[Co(C2O4)3]
iii. K3[Co(C2O4)2CI2]
iv. [Co(en)2CIBr]
How many structural isomers are possible with molecular formula C4H10O ?
One of the following rubbers is used in making oil seals, tank lining, etc.
(Yellow ppt) T X
Y. (Yellow ppt) + Z (pungent smellinggas)
If X gives green flame test. Then, X is :
The primary difference between the modern periodic table and Mendeleev's periodic table is:
Consider following reactions in equilbrium with equilibrium concentration 0.01M of every species
(I) PCl5(g)  PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)
(II) 2HI(g)  H2(g) + I2(g)
H2(g) + I2(g)
(III) N2(g) + 3H2 (g)  2NH3(g)
2NH3(g)
Extent of the reactions taking place is :
For moderation of the climate and body temperature of living beings, the responsible factor is:
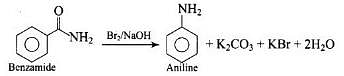
Hoffmann Bromamide Degradation reaction is shown by __________.
Eka silicon predicted by Mendeleev is which element:
Maximum number of compounds are known in the case of which inert gas?