AWES PGT Chemistry Mock Test - 2 - AWES TGT/PGT MCQ
30 Questions MCQ Test - AWES PGT Chemistry Mock Test - 2
On which date is International Safety Pin Day celebrated annually?
Rafah border crossing is located between which of the following?
Who has been selected as the next CMD of New India Assurance?
Mataberi Pera & Pachra, recently got a GI tag, belongs to which state?
Which of the following methods is most suitable to teach heterogeneous group of students for self placed learning?
Spontaneity is the hallmark of which of the following sessions?
'Education-of-all-in-schools-for-all' could be a tagline for which of the following?
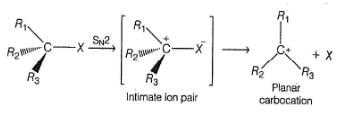
Consider the following reaction,

Q.
Which of the labeiled C—C bond formation is not possible in the above reaction?
In Tyndall effect the colloidally suspended particles:
We can obtain ethylamine by Hoffmann bromamide reaction. The amide used in this reaction is:
For the reversible reaction,
In a reaction vessel, [NO]= [O2]= 0.01 mol L-1 and [NO2]= 0.1 mol L-1 then above reaction is
Consider the following set of molecules.
The pairs of enantiomers are
From the stability constant (hypothetical values), given below, predict which is the strongest ligand:
A chemical reaction [2A] + [2B] + [C] → product follows the rate equation : then order of reaction is - [AIEEE-2002]
In oxygen difluoride (OF2) and dioxygen difluoride(O2F2), the oxygen is assigned an oxidation number of
What is the equilibrium constant for the reaction P4(s) + 5O2(g)  P4O10(s) :
P4O10(s) :
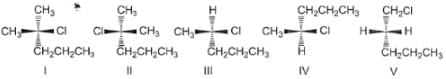
In SN1 reaction, racemisation occurs if the reaction occurs at a stereogenic centre, however, 50:50 mixture of enantiomers are rarely obtained, why?
The decreasing order of the repulsive interactions between various electron pairs is:
presence of extensive hydrogen bonding between water molecules leads to
What would be the major product in the following reaction?
At 25 ° C, the density of 18 MH2SO4 is 1.8 g cm3. Thus, mass percentage of H2SO4 in aqueous solution is
Direction (Q. Nos. 15 and 16) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
Q.
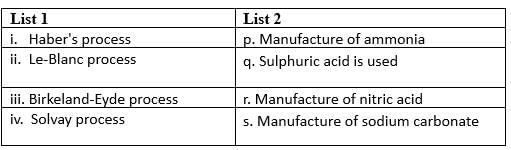
Match the Column I with Column II and mark the correct option from the codes given below.
Direction (Q. Nos. 18-20) This section contains a paragraph, each describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d).
Fluorine nitrate, is an oxidising agent, used as a rocket propellant.
this oxygen is different from other two)
Q. Possible structure of FONO2 is
Kf for water is 1.86 K kg mol-1. If your automobile radiator holds 1.0 kg of water, how many grams of ethylene glycol (C2H6O2) must you add to get the freezing point of the solution lowered to – 2.8°C ?
[AIEEE-2012]
In the body, message between two neurons and that between neurons to muscles is communicated through certain chemicals. These chemicals are the:
For a multi-electron atom, set of quantum numbers is given as
2,0,0,1/2 ; 2,0,0,-1/2
Q. Thus, the next higher allowed set of n and / quantum numbers for this atom in its ground state is
Which of the following reacts with alcohols to form esters besides carboxylic acids?
Probability (electron charge density) of bonding and anti-bonding molecular orbitals are given.
Select the correct probability,
Which scientist proposed that atomic number is more fundamental property of an element than its atomic mass?