UP PGT Chemistry Mock Test - 3 - UPTET MCQ
30 Questions MCQ Test - UP PGT Chemistry Mock Test - 3
Ca(HCO3)2 is strongly heated and after equilibrium is attained, temperature changed to 25° C.
Ca(HCO3)2(s)⇌CaO(s) + 2CO2 (g) + H2O(g)
Kp = 36 (pressure taken in atm)
Thus, pressure set up due to CO2 is
Kp = 36 (pressure taken in atm)
Thus, pressure set up due to CO2 is
Select the correct statement if -
E°Mg2+/Mg = - 2.4V, E°Sn4+/Sn2+ = 0.1 V, E°MnO4-,H+/Mn2+ = 1.5 V, E° I2/I- = 0.5 V Here,
Ferric hydroxide is a negative sol, which of the following electrolyte will coagulate it most:
In which of the following set of compounds oxidation number of oxygen is not (- 2)?
Uncertainty in position of a particle of 25 g in space is 10-5 m. Hence uncertainty in velocity (ms-1) is (Planck's constant h = 6.6 × 10-34 Js) [AIEEE- 2002]
Direction (Q. Nos. 13 and 14) This section contains 2 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. Which of the following species has/have oxidation number of the metal as + 6 ?
In a multi-electron atom, which of the following orbitals described by the three quantum numbers will have the same energy in the absence of magnetic and electric fields?
The substances which are added to stabilizes the emulsions are called:
In a mixture of A and B, components show negative deviation when [AIEEE-2002]
Adsorption isobar is a curve showing variation of adsorption ______.
One kg of carbon produces __________ kg of carbon dioxide.
Aqueous solution of which of the following compounds is used to detect carbon dioxide:
Which block of the periodic table contains the man made elements?
Arrange the electrons present in the 4d, 3d, 4p and 3p orbitals in order of increasing energies
In the third period of the periodic table the element having smallest size is
The rate of chemical reaction becomes double for every 10o rise in temperature because of
The solubility of fluorides is much less as compared to corresponding chlorides
According to Werner’s theory , the primary valences of the central atom
Consider the following experimental facts,
I. When Cl2 gas is passed into Kl solution containing CHCI3, violet colour appears in CHCI3 layer.
II. When Cl2 gas is passed into KBr solution containing CHCI3, orange colour appears in CHCI3 layer.
III. When Cl2 gas is passed into a solution containing KBr, Kl and KCI, containing CHCI3, violet colour appears in CHCI3 layer.
Select the correct experimental facts.
Volume occupied by one molecule of water (density = 1 g cm-3) is
Stability of (C—Cl) bond in chlorobenzene is sim ilar to (C—Cl) bond in
The principle involved in paper chromatography is:
Rank the following molecules increasing in order of relative rate of SN1 solvolysis with methanol and heat.
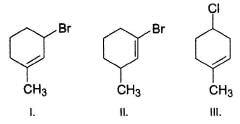
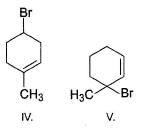
Find the number of stereoisomers for CH3 – CHOH – CH = CH – CH3.
In which of the following, is hydrogen peroxide not stored?
The number of photons emitted in 10 hours by a 60W sodium lamp (λ, of photon = 6000 Å)
[Take he = 12400 EvÅ, h = Planck's constant, c = speed of light]
An aqueous solution is 6% methanol, CH3OH, by mass with d = 0.988 g mL-1. Thus, molarity of CH3OH in this solution is




















