UP PGT Chemistry Mock Test - 10 - UPTET MCQ
30 Questions MCQ Test - UP PGT Chemistry Mock Test - 10
The density (in g mL-1) of a 3.60 M sulphuric acid solution that is 29% H2SO4(Molar mass = 98 g mol-1) by mass will be -
[AIEEE 2007]
Electron gain enthalpy values of noble gases are positive because:
amongst the following compounds, the optically active alkane having lowest molar mass is
[AIEEE 2004]
Which of the following is not a secondary pollutant
An element with atomic number will form a basic oxide________
A photon of 4000 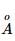 is used to break the iodine molecule, then the % of energy converted to the K.E. of iodine atoms if bond dissociation energy of I2 molecule is 246.5 kJ/mol is:
is used to break the iodine molecule, then the % of energy converted to the K.E. of iodine atoms if bond dissociation energy of I2 molecule is 246.5 kJ/mol is:
The pair of compounds, which cannot exist together in a solution is-
A red solid is insoluble in water. However, it becomes soluble if some KI is added to water. Heating the red solid in a test tube results in liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The red solid is
In a first order reaction, the concentration of the reactant, decreases from 0.8 M to 0.4 M in 15 minutes. The time taken for the concentration to change from 0.1 M to 0.025 M is -
[AIEEE-2004]
Direction (Q. Nos. 13 and 14) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
In the all oxyacids of phosphorus, each phosphorus atom is in sp3-hybridised state. All these acids contain P—OH bonds, the hydrogen atom of which are ionisable imparting acidic nature to the compound. The ‘ous’ acids (oxidation state of P is + 1 or + 3) also have P—H bonds in which hydrogens are not ionisable.
The presence of P—H bonds in these acids imparts reducing properties. The structure of some oxyacids are drawn below:
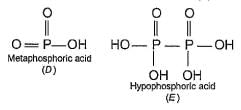
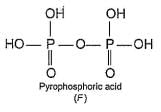
Q.
Although metaphosphoric acid is written as a monomer, it exists as a polymer (HPO3)n. The number of P—O—P bonds in cyclic trimetaphosphoric acid is
Assuming (2s-2p) mixing is not operative, the paramagnetic species among the following is
[JEE Advanced 2014]
Among the following elements, which one was initially placed in the 'rare earths' group in Mendeleev's Periodic Table?
Prussian blue is represented by KFex[Fe(CN)6]. Value of x is
Hardness of a water sample is 200 ppm CaCO3, Thus, molarity of CaCO3 is
A 20.0 litre vessel initially contains 0.50 mole each of H2 and I2 gases. These substances react and finally reach an equilibrium condition. Calculate the equilibrium concentration of HI if Keq = 49 for the reaction H2 + I2 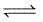 2HI.
2HI.
What is the increasing order of reactivity of the following in an E2 reaction with ethanolic KOH solution?
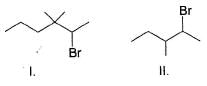
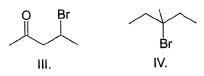
Approximately what percent of matter in the universe is believed to consist of hydrogen?
Comprehension Type
Direction (Q. Nos. 14-16) This section contains a paragraph, describing theory, experiments, data, etc. Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
An organic compound A (C7H16O) shows both enantiomerism and diastereomerism. Treatment of a pure enantiomer of A with Na2CrO4 /Dil. H2SO4 gives B (C7H14O) - Also A on dehydration with concentrated H2SO4 gives a single alkene C (C7H14). Ozonolysis of C followed by work-up with Zn-H2O gives D (C5H10O) as one of the product which gives racemic mixture on reduction with NaBH4.
Q.
The structure of A satisfying the above criteria is
Water has a maximum density at _____ degree centigrade.
What is the dominant intermolecular force or bond that must be overcome in converting liquid CH3CH2OH to vapours?
When Al2O3 is electrolysed ,cation and anions are discharged. For a given quantity of electricity,ratio of number of moles of Al and O2 gas is
What is wrong about enantiomers of 2-chloropropanoic acid?
As you move from top to bottom down the periodic table:
A 5.2 molal aqueous solution of methyl alcohol (CH3OH) is supplied. What is the mole fraction of methyl alcohol in the solution?
Direction (Q. Nos. 1-16) This section contains 16 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. The correct statement for the molecule Csl-3 is


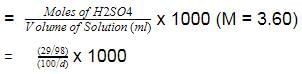

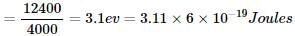
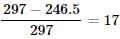
 Na2CO3 + H2O
Na2CO3 + H2O


















