EMRS PGT Chemistry Mock Test - 9 - EMRS MCQ
30 Questions MCQ Test - EMRS PGT Chemistry Mock Test - 9
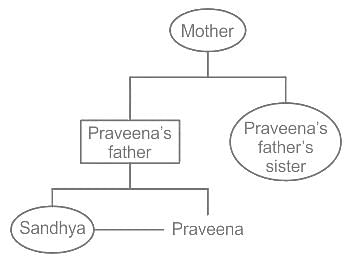
Sandhya is sister of Praveena. How is Praveena’s father’s sister’s mother related to Sandhya?
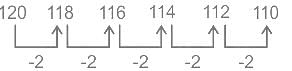
Find the next term in the given series.
120, 118, 116, 114, 112, ?
What is the intersection of a column and a row on a worksheet called?
A ______________ is a web site like any other, but it is intended to offer personal opinions of people on their hobbies, interests, commentaries, photo, etc.
PCI5 has a shape of trigonal bipyramid whereas, IF5 has a shape of square pyramid. It is due to
Direction (Q. Nos. 23 and 24) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
Q.
Match the species in Column I with the structure in Column II.
In the catalyzed decomposition of benzene diazonium chloride,
Half life period is found to be independent of the initial concentration of the reactant. After 10 min, the volume of N2 gas collected is 10 L and after the reaction is complete, it is 50 L. Hence, the rate constant of the reaction(in min-1) is
Which of the following statements about photochemical smog is wrong?
Which of the following processes does not involve a catalyst?
We know that the relationship between Kc and Kp is Kp = Kc (RT)Δn
What would be the value of Δn for the reaction NH4Cl (s) ⇔ NH3 (g) + HCl (g)
What is the electronic configuration of carbon in it’s excited state?
Which of the following will have the highest coagulating power for As2S3 colloids?
The atomic radii of Cu and Ag are 1.17 Å and 1.34 Å. The atomic radius of gold is expected to be
In a progressive set-up children with special needs:
Which of the following tools/methods does National Education Policy 2020 propose for assessment of children?
(i) Role plays
(ii) Group work
(iii) Portfolios
(iv) Projects
In the following question, four words are given out of which one word is incorrectly spelled. Find the incorrectly spelled word.
Improve the bracketed part of the sentence with the parts given below.
Q. He was (accepting) a great surprise on his birthday.
In the following question, a sentence has been given in Direct/Indirect speech. Out of the four alternatives suggested, select the one which best expresses the same sentence in the Indirect/Direct speech.
Q. He said, "I am doing the work."