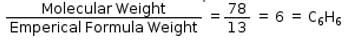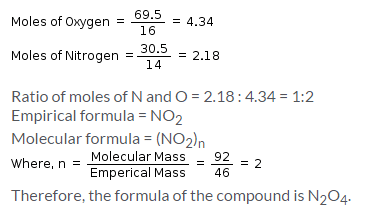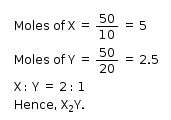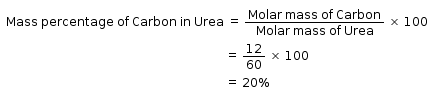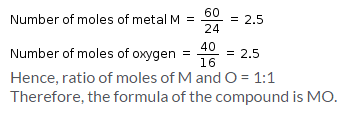Test: Empirical Formula & Mass Calculations - NEET MCQ
15 Questions MCQ Test - Test: Empirical Formula & Mass Calculations
Empirical formula of an organic compound is CH and its molecular mass is 78g/mol. What is its molecular formula?
A compound contains 69.5% oxygen and 30.5% nitrogen and its molecular mass is 92g/mol. The formula of that compound is:
Which formula represents the simplest whole number ratio of various atoms present in a compound?
What is the empirical formula of C4H8O2?
The empirical formula of an organic gas containing carbon and hydrogen is CH2. The mass of 1 litre of this organic gas is exactly equal to that of 1 litre of N2. Therefore, the molecular formula of organic compound is:
Which of the following compounds has the same empirical formula as that of glucose (C6H12O6)?
The simplest formula of a compound containing 50% of element X (atomic mass = 10g/mol) and 50% of element Y (atomic mass 20g/mol) is:
Formula mass is applicable in the case of:
What is the empirical formula of C2H2 and C6H6?
A compound has an empirical formula of C2HBr. If the molar mass of the compound is 314.7 g/mol, what is the molecular formula of the compound? (Atomic mass of Br = 80g/mol)
The empirical formula of an acid CH2O2 is:
What is the mass percentage of carbon in urea [CO(NH2)2]? (Molar mass of urea is 60g)
An oxide of metal M has 40% by mass of oxygen. Metal M has relative atomic mass of 24g/mol. The empirical formula of the oxide is:
The empirical formula of a compound is CH2Cl. If the molecular mass of the compound is 99 g, what is the molecular formula of the compound?
If empirical formula of a compound is C5H4 and the value of n is 2, then molecular formula of the compound is: