Test: Laws Of Chemical Combinations & Dalton's Atomic Theory - NEET MCQ
20 Questions MCQ Test - Test: Laws Of Chemical Combinations & Dalton's Atomic Theory
Which law is also known as Law of constant composition?
According to Dalton’s atomic theory chemical reactions involve:
100mL of gaseous hydrogen combines with 50 mL of gaseous oxygen to give 100mL of water vapours. This can be explained on the basis of:
According to the Avogadro’s law, equal volumes of gases at the same temperature and pressure should contain:
The distinction between atoms and molecules was made by:
Law of definite proportions is given by:
According to Avogadro’s law at the same temperature and pressure:
Law of constant composition does not hold good for:
Laws of chemical combinations can be explained on the basis of:
What is incorrect about the Law of conservation of mass?
A statement which is not a part of Dalton’s atomic theory is:
Natural sample of cupric carbonate contains 51.35% of copper, 9.74% of oxygen and 38.91% of carbon. Synthetic sample of the compound will contain:
A compound prepared by any method contains the same elements in the fixed ratio by mass. The given statement is known as:
At what conditions Gay Lussac’s law of gaseous volumes is considered?
Law of conservation of mass was given by:
Which of the following statements illustrate the law of multiple proportions?
If 6.3 g of NaHCO3 are added to 15.0 g CH3COOH solution, the residue is found to weigh 18.0 g. What will be the mass of CO2 released in the reaction?
When hydrogen combines with oxygen, it produces water and hydrogen peroxide. This illustrates:
4.88 g of KClO3 when heated, produces 1.92 g of O2 and 2.96 g of KCl. Which of the following statements is true regarding the experiment?
Zinc sulphate contains 22.65% zinc and 43.9% water of crystallisation. If the law of constant proportions is true then the weight of zinc required to produce 20 g of the zinc sulphate crystals will be



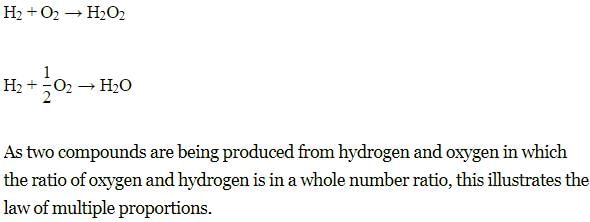
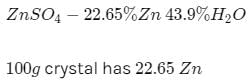 20 g of zinc sulphate
20 g of zinc sulphate










