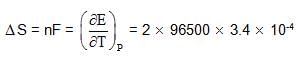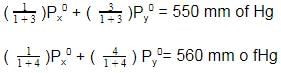Bihar STET Paper 2 Chemistry Mock Test - 2 - Bihar PGT/TGT/PRT MCQ
30 Questions MCQ Test - Bihar STET Paper 2 Chemistry Mock Test - 2
What is cell entropy change of the following cell?
Pt(s) | H2(g) | CH3COOH, HCl || KCl (aq) |Hg2Cl2| (s) | Hg
P = 1 atm 0.1M 0.1M
Emf of the cell is found to be 0.045 V at 298 K and temperature coefficient is
3.4 x10–4 VK–1
Given Ka (CH3COOH) = 10–5 M
Powdered substances are more effective adsorbents than their crystalline forms because:
Correct decreasing order of dipole moment of CH3F, CH3CI and CH3Br is
We can obtain ethylamine by Hoffmann bromamide reaction. The amide used in this reaction is:
The rate constant of the reaction at temperature 200 K is 10 times less than the rate constant at 400 K. The activation energy of the reaction is
Consider the following transformations.
Q. Which reaction sequence will bent bring about the above transformation?
The equilibrium constant for the reaction
CO(g) + H2O(g) 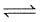 CO2(g) + H2(g) is 3 at 500 K. In a 2 litre vessel 60 gm of water gas [equimolar mixture of CO(g) and H2(g)] and 90 gm of steam is initially taken.
CO2(g) + H2(g) is 3 at 500 K. In a 2 litre vessel 60 gm of water gas [equimolar mixture of CO(g) and H2(g)] and 90 gm of steam is initially taken.
What is the equilibrium concentration of H2(g) at equilibrium (mole/L) ?
Two liquids X and Y form an ideal solution At 300 K, vapour pressure of the solution containing 1 mol of X and 3 mol of Y is 550 mmHg. At the same temperature, if 1 mol of Y is further added to this solution, vapour pressure of the solution increases by 10 mmHg. Vapour pressure (in mmHg) of X and Y in their pure states will be, respectively -
[AIEEE 2009]
At what minimum atomic number, a transition from n = 2 to n = 1 energy level results in the emission of X-rays with wavelength 3.0 x 10-8 m?
Which among the following is adsorbed greatly by activated charcoal?
In oxygen difluoride (OF2) and dioxygen difluoride(O2F2), the oxygen is assigned an oxidation number of
Which of the following types of octahedral complexes will exhibit geometrical isomerism (where, M = metal, a, b = achiral ligands)?
Entropy change when 2 moles of an ideal gas expands reversibly from an initial volume of 1 dm3 to a final volume of 10 dm3 at a constant temperature of 298 K is
Comprehension Type
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)
Passage I
A constant current of 30 A is passed through an aqueous solution of NaCl for a time of 1.00 h.
Thus NaOH formed due to electrolysis is
Once the equilibrium is reached under given condition:
Given, ΔfH° of HCI (g) is - 22 . 10 kcal mol-1 and ΔSolutionH° (heat of solution) of HCI (g) is - 17.9 kcal mol-1. Thus, ΔfH ° of Cl- (aq) is
The ≈ pH of the neutralisation point of 0.1 N ammonium hydroxide with 0.1 N HCl is
In an experiment conducted on a hungry chimpanzee, some bananas were kept outside its cage, but beyond its reach. Some sticks were also kept in its cage. After several unsuccessful attempts to reach out to the bananas, the chimpanzee pondered over the problem. Then, he picked up a stick and pulled the bananas towards itself. In this case, learning took place by
_________ is the study of cognitive development that is concerned with basic mechanics of learning.
How does NEP 2020 propose to address the flexibility in subject choices for students in higher education?
What is the purpose of the National Testing Agency (NTA) in the context of NEP 2020?
Which of the following is a socialization agency?
Madam Soni found that most of the students this year performed better in exams than the last year. This may be due to:
Mrs. Sharma can impart learning with better skill and results because she possesses
Who has been appointed as the chairperson and independent director of PayU Payments Private Ltd?
In 1992, Rio Declaration was conducted on the issue of environment and development. In this conference, an action plan was framed for sustainable development. What was the name of that action plan?
Ramesh is studying about the various geographical barriers of the world. He also reads about the Palk Strait. The Palk Strait lies between
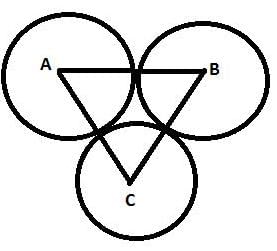
The three equal circles touches each other externally. If the centres of these circles be A, B, C then ΔABC is ________