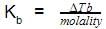Bihar STET Paper 2 Chemistry Mock Test - 9 - Bihar PGT/TGT/PRT MCQ
30 Questions MCQ Test - Bihar STET Paper 2 Chemistry Mock Test - 9
A " 1/4 HP" electric motor uses 187 W of electrical energy while delivering 35 J of work each second. How much energy must be dissipated in the form of friction (heat)?
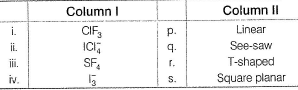
Direction (Q. Nos. 23 and 24) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
Q.
Match the species in Column I with the structure in Column II.

Photoelectric effect established that light
The exothermic formation of ClF3 is represented by the equation :
Cl2(g) + 3F2(g)  2ClF3(g) ; ΔH = -329 kJ
2ClF3(g) ; ΔH = -329 kJ
Which of the following will increase the quantity of ClF3 in an equilibrium mixture of Cl2, F2 and ClF3 :
Which compound given below has the highest solubility in water ?
One Integer Value Correct Type
This section contains 1 questions, when worked out will result in an integer value from 0 to 9 (both inclusive)
Q. Certain brine solution has 3.87% NaCI by mass with density 1.036 g mL-1. How many millilitre of this solution should be evaporated to obtain 0.32 g of NaCI?
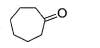
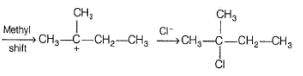
What is the correct structure for the major compound produced by the following reaction sequence?
Number of electrons having l + m value equal to zero in 26Fe may be
What is the oxidation state of Rb in RbO2?
The phenomenon in which adsorption and absorption takes place simultaneously is called?
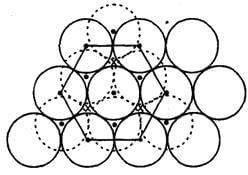
In hexagonal close packing of sphere in three dimensions.
Giant ionic structures is also name given to
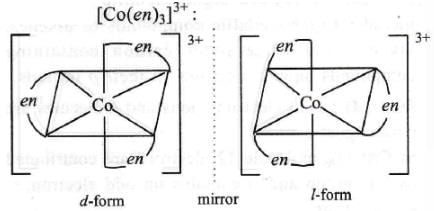
Which of the following coordination compounds would exhibit optical isomerism?
The molal elevation constant is the ratio of the elevation in B.P to:
Newland arranged elements in increasing order of atomic weights and noted that every eighth element had properties similar to:
The drug which can act both as an analgesic and antipyretic is
What is the product formed in the following reaction?
Which of the following could result as a product in the aldol condensation reaction?
Statement-1: In electrochemical cell, we cannot use KCI in the salt bridge if anodic or cathodic compartment consists of Ag+ of Pb2+ ion.
Statement-2: Salt bridge is employed to maintain the electrical neutrality and to minimize the liquid-liquid junction potential.
Identify the model of inclusive education in which a student is included during regular education classroom instruction to provide the student appropriate interaction with non-disabled peers. The student is given shortened assignment.
Sita is a visual learner, i.e. she learns best by seeing. She may get the least help from
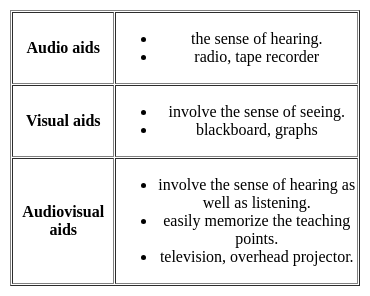
Audio-Visual aids help the teacher because :
Which of the following methods are learner-centered teaching methods?
(A) Active learning
(B) Co-operative learning
(C) Inductive teaching and learning
(D) Passive learning
Choose the most appropriate answer from the options given below:
Arrange the following teaching steps in order:
(i) Relate the present knowledge with previous one
(ii) Evaluation
(iii) Reteaching
(iv) Formulating instructional objectives
(v) Presentation of instructional materials
The idea of showing a sample of a railway ticket in EVS book is to
What is the area of the largest square that can be made in a rectangle with 10 meters length and 4 meters width?
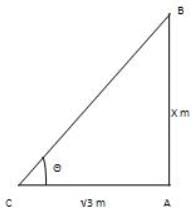
At a moment, the shadow's length of a shaft is √3 times the stature of the shaft. The edge of rise of the sun is:
Study the following carefully and answer the given question.
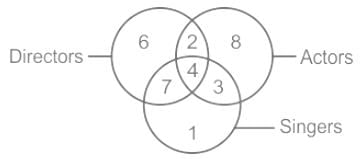
Which region denotes singers, who are directors but not actors?