Test: Addition & Condensation Polymers (Old NCERT) - JEE MCQ
Test Description
10 Questions MCQ Test - Test: Addition & Condensation Polymers (Old NCERT)
Test: Addition & Condensation Polymers (Old NCERT) for JEE 2025 is part of JEE preparation. The Test: Addition & Condensation Polymers (Old NCERT) questions and answers have been prepared
according to the JEE exam syllabus.The Test: Addition & Condensation Polymers (Old NCERT) MCQs are made for JEE 2025 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Addition & Condensation Polymers (Old NCERT) below.
Solutions of Test: Addition & Condensation Polymers (Old NCERT) questions in English are available as part of our course for JEE & Test: Addition & Condensation Polymers (Old NCERT) solutions in
Hindi for JEE course.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free. Attempt Test: Addition & Condensation Polymers (Old NCERT) | 10 questions in 15 minutes | Mock test for JEE preparation | Free important questions MCQ to study for JEE Exam | Download free PDF with solutions
Detailed Solution for Test: Addition & Condensation Polymers (Old NCERT) - Question 1
Test: Addition & Condensation Polymers (Old NCERT) - Question 2
A synthetic polymer which resembles natural rubber is:
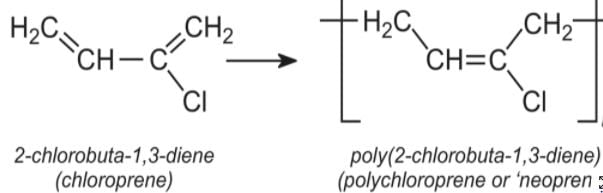
Detailed Solution for Test: Addition & Condensation Polymers (Old NCERT) - Question 2
Test: Addition & Condensation Polymers (Old NCERT) - Question 3
Which type of polymer will be formed if the susbstituent group is –C6H5
Detailed Solution for Test: Addition & Condensation Polymers (Old NCERT) - Question 3
Test: Addition & Condensation Polymers (Old NCERT) - Question 4
Vulcanisation is used in processing of:
Detailed Solution for Test: Addition & Condensation Polymers (Old NCERT) - Question 4
Detailed Solution for Test: Addition & Condensation Polymers (Old NCERT) - Question 5
Detailed Solution for Test: Addition & Condensation Polymers (Old NCERT) - Question 6
Test: Addition & Condensation Polymers (Old NCERT) - Question 7
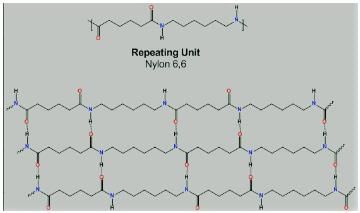
Which intermolecular force is present in Nylon 6,6?
Detailed Solution for Test: Addition & Condensation Polymers (Old NCERT) - Question 7
Test: Addition & Condensation Polymers (Old NCERT) - Question 8
Polymers having ester linkage are called:
Detailed Solution for Test: Addition & Condensation Polymers (Old NCERT) - Question 8
Detailed Solution for Test: Addition & Condensation Polymers (Old NCERT) - Question 9
Test: Addition & Condensation Polymers (Old NCERT) - Question 10
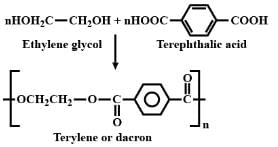
Condensation polymerisation of ethylene glycol and terepthalic acid gives:
Detailed Solution for Test: Addition & Condensation Polymers (Old NCERT) - Question 10
Information about Test: Addition & Condensation Polymers (Old NCERT) Page
In this test you can find the Exam questions for Test: Addition & Condensation Polymers (Old NCERT) solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Addition & Condensation Polymers (Old NCERT), EduRev gives you an ample number of Online tests for practice
Download as PDF