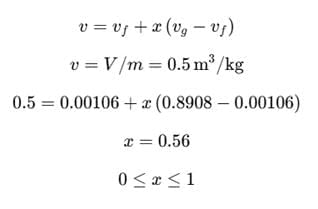Test: Change of Phase - NEET MCQ
10 Questions MCQ Test - Test: Change of Phase
A student added a drop of red food coloring to 4 beakers of water.Each beaker contained 100 ml of a different temperature water. The student recorded how long it took each beaker to mix completely (without stirring). The following table shows her results:
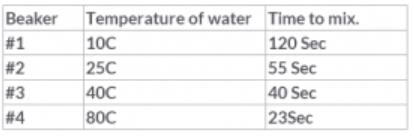
What conclusion should the student make from this experiment? Particles are moving

Which diagram is the best depiction of the direction of flow of diffusion?
The temperature and pressure at which all three phases of a substances coexist is called
An increase in temperature in a liquid would cause a phase change to which of the following?
What happens to the volume of the substance when the temperature increases?
Skating is possible on snow due to the formation of water below the skates. This water below skates comes as result of
If a substance of mass m undergoes a change of state (like melting or boiling) without a change in temperature, the amount of heat Q required is given by:
A rigid container of volume 0.5 m³ contains 1.0 kg of water at 120°C (vf = 0.00106 m³/kg, vg = 0.8908 m³/kg). The state of water is