Test: Scattering of Alpha Particles - JEE MCQ
10 Questions MCQ Test - Test: Scattering of Alpha Particles
Which of the following is/are deduced from the Rutherford’s scattering experiment?
(1) There are neutrons inside the nucleus.
(2) The sign of the charge of the nuclei is the same as the sign of alpha particles.
(3) Electrons are embedded in the nucleus.
(1) There are neutrons inside the nucleus.
(2) The sign of the charge of the nuclei is the same as the sign of alpha particles.
(3) Electrons are embedded in the nucleus.
In scattering, the impact parameter b is defined as the:
The alpha particle scattering experiment was carried out by:
We know that the Rutherford model of the atom is superior to the Thompson model because when alpha particles are scattered from atoms:
In Rutherford’s experiment, a thin gold foil was bombarded with alpha particles. According to Thomson’s “plum-pudding” model of the atom, what should have happened?
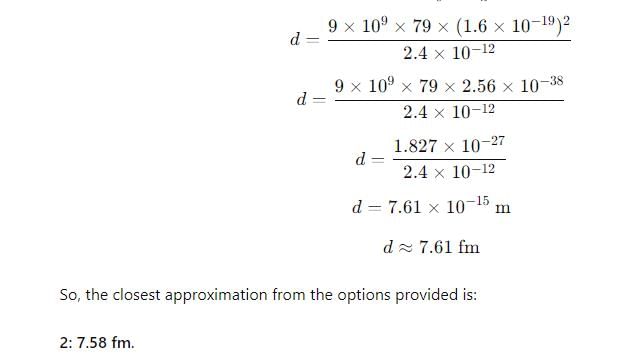
The distance of closest approach when a 15.0 MeV proton approaches gold nucleus (Z = 79) is
The targets used in the alpha particle atomic experiments in the early 1900’s was:
Rutherford’s experiments on scattering of alpha particles proved that:
Number of alpha particles N scattered at an angle θ during Rutherford’s alpha scattering experiment is :



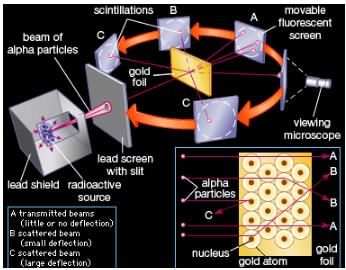 Physicist Ernest Rutherford established the nuclear theory of the atom with his gold-foil experiment. When he shot a beam of alpha particles at a sheet of gold foil, a few of the particles were deflected. He concluded that a tiny, dense nucleus was causing the deflections.
Physicist Ernest Rutherford established the nuclear theory of the atom with his gold-foil experiment. When he shot a beam of alpha particles at a sheet of gold foil, a few of the particles were deflected. He concluded that a tiny, dense nucleus was causing the deflections.















