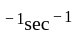MP Police SI (Technical) Mock Test - 7 - MP Police SI MCQ
30 Questions MCQ Test - MP Police SI (Technical) Mock Test - 7
If a simple harmonic oscillator has got a displacement of 0.02m and acceleration equal to 2m/s2 at any time, the angular frequency of the oscillator is equal to ___________
If 75% of a number is added to 75, then the result is the number itself. Find the number.
Two bodies at different temperatures 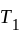 and
and 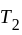 if brought in thermal contact:
if brought in thermal contact:
In a solid ' ' having the
' having the  structure, '
structure, ' ' atoms occupy the corners of the cubic unit cell. If all the face centred atoms along one of the axes are removed, then the resultant stoichiometry of the solid is:
' atoms occupy the corners of the cubic unit cell. If all the face centred atoms along one of the axes are removed, then the resultant stoichiometry of the solid is:
The gravitational force between two masses kept at a certain distance is 'P' Newton. The same two masses are now kept in water and the distance between them are same. The gravitational force between these two masses in water is 'Q' Newton then:
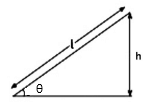
A smooth inclined plane is inclined at an angle 9 with the horizontal as shown in the above figure. A body starts from rest and slides down the inclined surface. The time taken by the body to reach the bottom is:
Which one of the following has largest number of isomers?
(R = alkyl group, en = ethylenediamine)
Some minerals contain high percentage of a particular metal. These minerals are known as _________.
An ideal gas heat engine operates in Carnot's cycle between 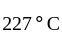 and
and 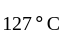 It absorbs
It absorbs 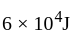 at high temperature. The amount of heat converted into work is _________.
at high temperature. The amount of heat converted into work is _________.
Which of the following will not show geometrical isomerism?
Which of the following ore is concentrated using group 1 cyanide salt?
If 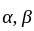 are the roots of the equation
are the roots of the equation 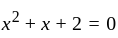 , then what is
, then what is 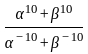 equal to?
equal to?
Find the total amount on a principal of Rs. 300 in 2 years at 5% per annum compound interest?
Which of the following is the simplest member of organic compounds?
Direction: In the following question two equation numbered I and II are given. Solve the equation and answer the question:
I. 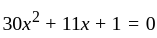
II. 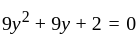
An ideal refrigerator has a freezer at a temperature of 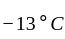 .The coefficient of performance of the engine is
.The coefficient of performance of the engine is 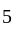 . The temperature at which heat is rejected will be:
. The temperature at which heat is rejected will be:
A certain sum of money fetched an interest of Rs. 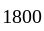 at
at 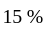 per annum simple interest for
per annum simple interest for 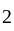 years. Find the sum.
years. Find the sum.
Two persons are standing on opposite ends of a field of length 1500 meters. If they are running towards each other at 10 km/hr and 20 km/hr respectively, after how much time will they meet each other?
Which instrument is used to measure the density of milk?
Which one of the following is a square root of 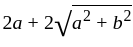 , where
, where 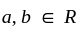 ?
?
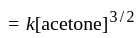 acetone then unit of rate constant and rate of reaction respectively is
acetone then unit of rate constant and rate of reaction respectively isA gas filled balloon moves up because ___________.
Which of the following statements is/are true?
The reaction; 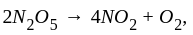 shows an increase in concentration of
shows an increase in concentration of 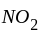 by
by 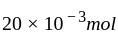 litre
litre 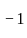 in 5 second.
in 5 second.
Calculate
(a) rate of appearance of 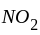 ,
,
(b) rate of reaction and
(c) rate of disappearance of  .
.
The value of p for which the sum of the squares of the roots of the equation x2 - (p - 2)x - p + 1 = 0 is minimum will be:
Two bodies in contact are said to be in thermal equilibrium when:


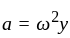
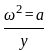
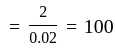
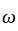
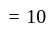 rad/s
rad/s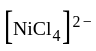 have tetrahedral shape while
have tetrahedral shape while 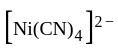 have square planner structure.
have square planner structure.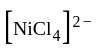 reveals that the species is paramagnetic having two unpaired electrons i.e.,
reveals that the species is paramagnetic having two unpaired electrons i.e., 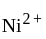 is
is 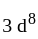 . The four electron pairs by
. The four electron pairs by 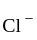 ligand leads to
ligand leads to 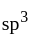 - hybridization in
- hybridization in 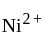 and do not disturb
and do not disturb 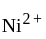 configuration to give tetrahedral geometry.
configuration to give tetrahedral geometry.
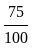
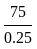
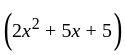 .
.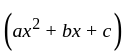
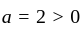
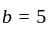 and
and 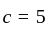
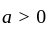 , then the minimum value of equation
, then the minimum value of equation 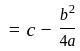
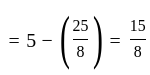
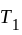 and
and 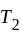 are brought in thermal contact, heat is said to flow from the body at higher temperature to the body at lower temperature until an equilibrium is obtained, i.e., until a point at which the temperatures of both the bodies becomes the same. The equilibrium temperature turns out to be the same as the mean temperature, which can be denoted as
are brought in thermal contact, heat is said to flow from the body at higher temperature to the body at lower temperature until an equilibrium is obtained, i.e., until a point at which the temperatures of both the bodies becomes the same. The equilibrium temperature turns out to be the same as the mean temperature, which can be denoted as 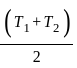 only when thermal capacities of the two bodies are the same.
only when thermal capacities of the two bodies are the same.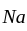 in
in 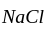 has eight corner and six face atoms and thus, if we remove face centred atom of one axis, then two face atoms are removed.
has eight corner and six face atoms and thus, if we remove face centred atom of one axis, then two face atoms are removed. 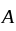 is at
is at 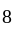 corners
corners 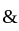 at
at 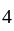 face centers.
face centers. 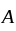 atoms per unit cell
atoms per unit cell 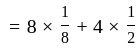
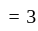
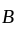 present in all octahedral voids, thus number of
present in all octahedral voids, thus number of 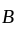 atoms per unit cell
atoms per unit cell 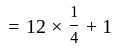
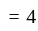
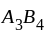 .
.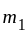 and
and 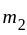 is proportional to the product of their masses and the distance between them is the square of
is proportional to the product of their masses and the distance between them is the square of 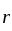 . Is inversely proportional to force.
. Is inversely proportional to force.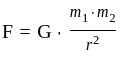
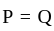
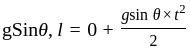
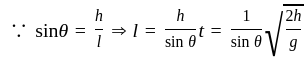
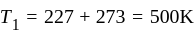
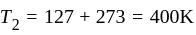
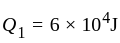 .
.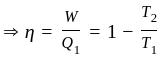
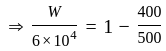
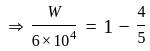
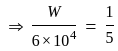
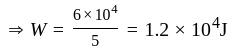
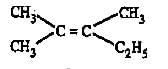
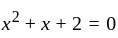 comparing with
comparing with 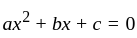
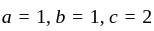
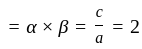
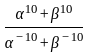
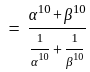
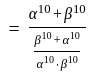
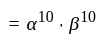
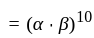
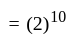
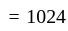
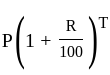
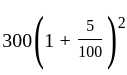
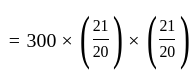
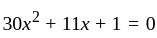
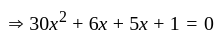
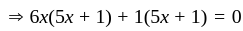
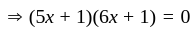
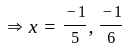
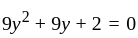
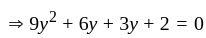
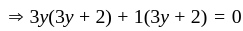
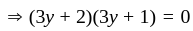
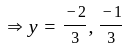
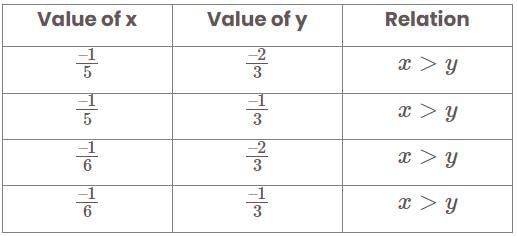
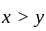
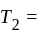
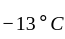
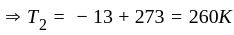
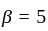
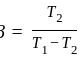
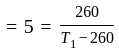
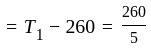
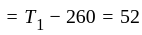
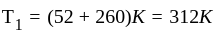
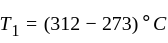
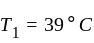
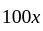
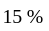
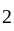
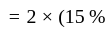
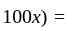
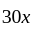
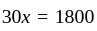
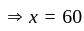
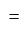
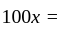
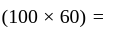
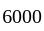
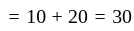 km/hr
km/hr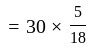 m/s
m/s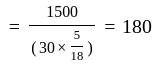 seconds
seconds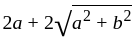
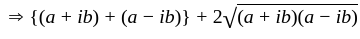
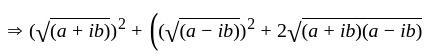
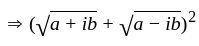
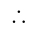 The square root of
The square root of 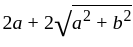 is
is 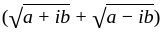 .
.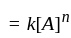
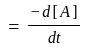
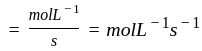
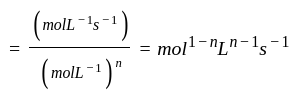
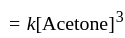
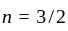
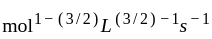
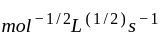
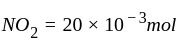 litre
litre 
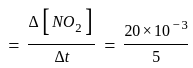
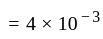 mol litre
mol litre 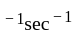
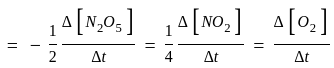
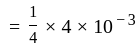
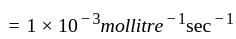
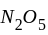
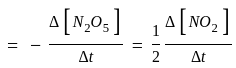
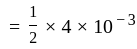
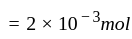 litre
litre