Test: Nomenclature of Organic Compounds (27 August) - JEE MCQ
10 Questions MCQ Test - Test: Nomenclature of Organic Compounds (27 August)
Which of the following compounds will exhibit cis-trans isomerism?
For which of the following parameters the structural isomers C2H5OH and CH3OCH3 would be expected to have the same values?
[AIEEE 2004]
Which of the following statements are correct?
I. A pair of positional isomers differs in the position of the same functional group.
II. A pair of. structural isomers have the same relative molar mass.
Ill. A pair of functional group isomers belongs to different homologous series.
II. A pair of. structural isomers have the same relative molar mass.
Ill. A pair of functional group isomers belongs to different homologous series.
How many alcohols are structural isomers with the formula: C5H11OH?
Which of the following has three different stereoisomers?
Direction (Q. Nos. 14-18) This section contains 5 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q.
Which compound below can have a non-polar steroisomers ?
Which compound below exhibit stereolsomerism?
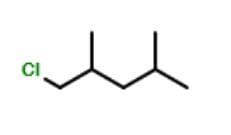
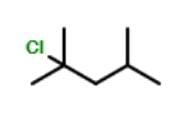
Consider the following free radical chlorination reaction.
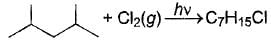
The correct statement about product(s) is/are
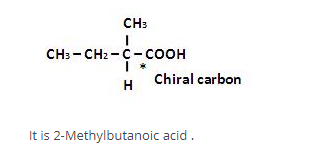
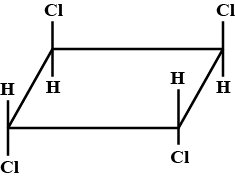
The correct statement(s) about the compound given below is/are
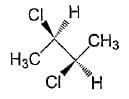
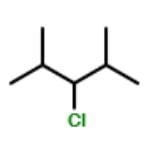
Which of the following statements is/are correct regarding the below compound?





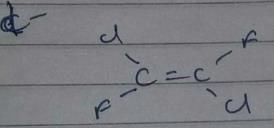
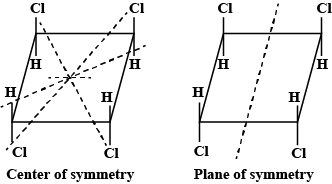
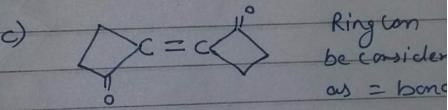 However, there is no such arrangement in structure i, it won’t have any non polar structure.
However, there is no such arrangement in structure i, it won’t have any non polar structure.