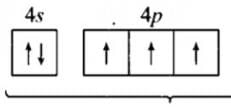
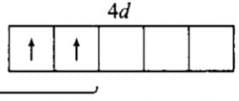
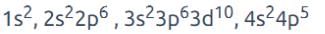AWES TGT Chemistry Mock Test - 3 - AWES TGT/PGT MCQ
30 Questions MCQ Test - AWES TGT Chemistry Mock Test - 3
Which institution has launched the ‘Mission 50K-EV4ECO’?
Which Indian state's delegation is heading to Switzerland for the World Economic Forum to showcase its vision of becoming a trillion-dollar economy?
Recently, who was honored with the ‘International Culture Award 2024’?
According to NEP 2020, what is the role of technology in education?
Under NEP 2020, what is the target for the Gross Enrollment Ratio (GER) in higher education by 2035?
For the four successive transition elements (Cr, Mn, Fe and Co), the stability of + 2 oxidation state will be there in which of the following order?
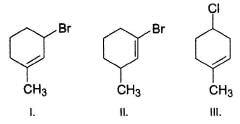
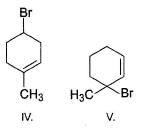
Rank the following molecules increasing in order of relative rate of SN1 solvolysis with methanol and heat.
During dehydration of alcohols to alkenes by heating with conc. H2SO4 the initiation step is-
[AIEEE-2003]
Yellow colour of NaCl crystals in sodium vapour is due to
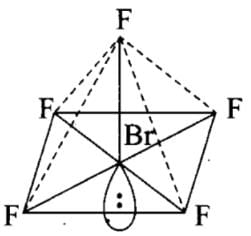
Which of the following has a square pyramidal shape?
In the modern periodic table, which period contains 32 elements?
Volume occupied by one molecule of water (density = 1 g cm-3) is
When a chemical bond is formed, there is decrease in
If Ksp for HgSO4 is 6.4 × 10-5, then solubility of this substance in mole per m3 is
The correct order of in creasing order of radiiions Br- , F-, O2- and S2- is as follow
A hydrocarbon X is optically. X upon hydrogenation gives an optically inactive alkane Y. Which of the following pair of compounds can be X and Y respectively?
Enthalpies of formation of CO(g) , CO2 (g) , N2O (g) and N2O4 (g) are -110, - 393, 81 and 9.7 kJ mol-1. Thus, ΔrU for the reaction at 298 K is,
What is relative reactivity of secondary versus primary hydrogens in free radical bromination of n-butane if the ratio of 1-bromo to 2-bromobutane formed is 7 : 39?
Which type of solids are held by weak dispersion forces?
Which of the following angle corresponds to sp2 hybridisation?
Which one of the following statement(s) is/are incorrect for lanthanides?
Adsorption isobar is a curve showing variation of adsorption ______.