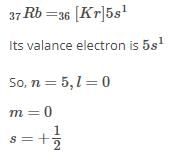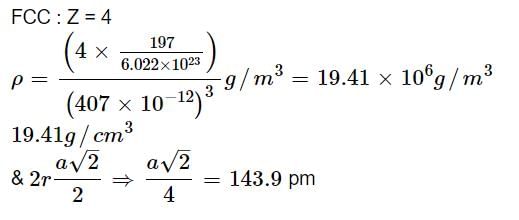AWES TGT Chemistry Mock Test - 6 - AWES TGT/PGT MCQ
30 Questions MCQ Test - AWES TGT Chemistry Mock Test - 6
Which city hosted the ‘National Conference on Water Sufficient Panchayat, Clean & Green Panchayat and Healthy Panchayat’?
To make assessment 'a useful and interesting process', one should be careful about:
According to NEP‐2020, National Research Foundation (NRF) may be established to
Q.
Statement I Cis-1,4-dichlorocyclobutane is optically inactive.
Statement II It possesses plane of symmetry.
[Ti (H2O)6]3+ absorbs green and yellow region part of visible light. Then the transmitted colour of the compound is
Chlorine, bromine and iodine when combined with oxygen, have oxidation numbers
Among the following the element with highest first ionization energy is:
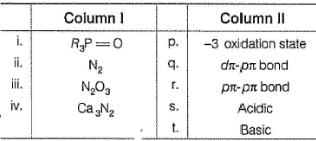
Match the Column I with Column II and mark the correct option from the codes given below :
For the process, and 1 atmosphere pressure, the correct choice is
[JEE Advanced 2014]
A hydrocarbon (R) has six membered ring in which there is no unsaturation. Two alkyl groups are atttached to the ring adjacent to each other. One group has 3 carbon atoms with branching at 1st carbon atom of chain and another has 4 carbon atoms. The larger alkyl group has main chain of three carbon atoms of which second carbon is substituted. Correct IUPAC name of compound (R) is
The correct set of four quantum numbers for the valence electron of rubidium atom (Z = 37) is
[JEE Main 2013]
Chemical species present in the environment are either naturally occurring or generated by human activities. Their interrelation with the surroundings is called:
Which of the following is true regarding a SN1 reaction?
Hydrogen has tendency to gain one electron to acquire helium configuration, in this respect it resembles:
Valence shell MO electronic configuration of a diatomic species is shown
* is for anti-bonding molecular orbital (MO).
Q. Divalent cation of this species
The number of atoms in 0.1 mol of a triatomic gas is
Electron affinity reflects the ability of an atom to accept an electron. Which is true of the alkaline earths?
For the reaction
A(g) + 2B(g) 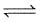 C(g) + D(g); Kc = 1012 If the initial moles of A, B, C and D are 0.5, 1, 0.5 and 3.5 moles respectively in a one litre vessel. What is the equilibrium concentration of B ?
C(g) + D(g); Kc = 1012 If the initial moles of A, B, C and D are 0.5, 1, 0.5 and 3.5 moles respectively in a one litre vessel. What is the equilibrium concentration of B ?
Gold crystallizes in a face centered cubic lattice. If the length of the edge of the unit cell is 407 pm, calculate the density of gold as well as its atomic radius assuming it to be spherical. Atomic mass of gold = 197 amu.
Direction (Q. Nos. 18 and 19) This section contains 2 questions. when worked out will result in an integer from 0 to 9 (both inclusive)
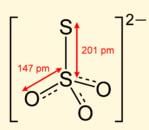
Q. S2O32- has two types of sulphur atoms. What is the difference in the oxidation states of two types of sulphur atoms?
How many valence electrons does a carbon atom have?
The compound
product,
here product is
[AIEEE-2002]
The compound used in enrichment of uranium for nuclear power plant is
Gadolinium belongs to 4f series. Its atomic number is 64. Which of the following is the correct electronic configuration of gadolinium?
What volume of 0.2 M NH4Cl solution should be added to 100 ml of 0.1 M NH4OH solution to produce a buffer solution of pH = 8.7 ?
Given : pKb of NH4OH = 4.7 ; log 2 = 0.3
Direction (Q. Nos. 18) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
Q. Match the entries in Column I with correctly related quantum number(s) in Column II