AWES TGT Chemistry Mock Test - 7 - AWES TGT/PGT MCQ
30 Questions MCQ Test - AWES TGT Chemistry Mock Test - 7
Which Union Ministry is associated with the Cinematograph (Amendment) Bill, 2023?
Recently, which state government has decided to repeal the Muslim Marriage and Divorce Registration Act, 1935?
What is the name of the world's first wooden satellite set to be launched by NASA and JAXA?
In which event did Jyothi Yarraji win the gold medal at the Asian Indoor Athletics Championship?
Which of the following tools/methods does National Education Policy 2020 propose for assessment of children?
(i) Role plays
(ii) Group work
(iii) Portfolios
(iv) Projects
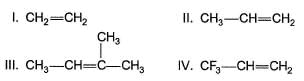
An alkene on treatment with meta chloro perbenzoic acid undergoes epoxidation reaction to give oxirane. What is the correct order of increasing reactivity of the following in the above mentioned epoxidation reaction?
At what temperature white phosphorous changes to red phosphorous?
Predict the major organic product in the following reaction.
Which of the following structures represents a chiral compound ?
Which of the following is prepared by cyanamide process?
Provide the structure of the major organic product given in the following reaction.
Rate of reaction can be expressed by Arrhenius equation as k = Ae–E/RT , In this equation, E represents
[AIEEE 2006]
The ionisation isomer of [Cr(H2O)4 CI(NO2)]CI is
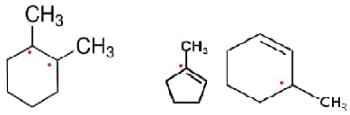
How many assymmetric carbon atoms are present in
(i) 2-Dimethyl cyclohexane
(ii) 3-Methyl cyclopentene
(iii) 3-Methylcyclohexene
Which of the following is least likely to form turbidity with HCI in the presence of ZnCI2 at room temperature?
In a 0.2 molal aqueous solution of a weak acid HX the degree of ionization is 0.3 . Taking kƒ for water as 1.85, the freezing point of the solution will be nearest to _
[AIEEE-2003]
In the following reaction,
Q.
The major final product is
In which of the following reactions, increase in the pressure at constant temperature does not affect the moles at equilibrium.
Statement Type
Direction (Q. Nos. 16 and 17) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Consider the following two bromides I and II, undergoing solvolysis reaction in boiling ethanol :
Statement I : I is less reactive than II in the given solvolysis reaction.
Statement II : Resonance stabilisation available with the intermediate formed from II is the important driving force.
Which of these is not released from burning fossil fuels?
If for the cell, Zn(s) + Cu2+(ag) Cu(s) + Zn2+ (ag)entropy change ΔS° is 96.5 JK-1 mol-1, then temperature coefficient of the emf of a cell is
How many grams of water would you add to 1.38 moles of CH3OH in 1 kg water to reduce the molality to 1.00 molal CH3OH(aq) ?
Addition of bromine on propene in the presence of brine yields a mixture of
Passage
Phosphorus was discovered by Brand (1669), Scheele isolated from bone ash and Lavoisier proved its elemental nature (1777). The principal minerals are phosphate rock, fluoroacetate, and chloroacetate. Phosphorus is prepared by the direct reduction of phosphorite by carbon in the presence of silica. It exists in different allotropic forms such as yellow or white, red, a-black,f3-black, etc. White P is most reactive, poisonous, glows in dark, and readily catches fire due to unstable discrete P4 molecules. Red P is inert, non-poisonous, does not glow, etc., due to its polymeric structure. a-black, f3 -black allotropes are also chemically inert, do not ignite at normal temperature. It has a layer structure like graphite and acts as a conductor.
Q. The allotrope of phosphorus that has layer lattice-like graphite is:
Consider a reversible isentropic expansion of 1.0 mole of an ideal monoatomic gas from 25°C to 75°C. If the initial pressure was 1.0 bar, final pressure is
H2 gas is adsorbed on the metal surface like tungsten. This follows........ order reaction –
[AIEEE-2002]



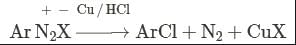
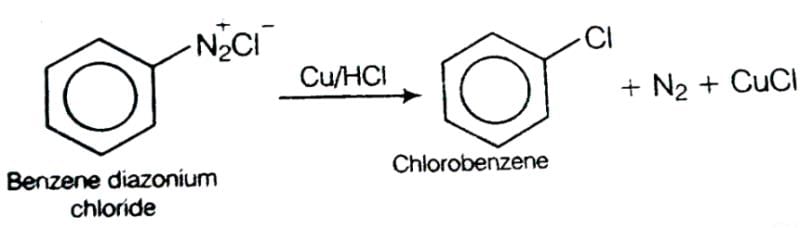 This reaction is called Gattermann reaction. In this reaction, Cl, Br and CN can be introduced into the benzene ring by simply treating diazonium salts with HCl, HBr, KCN. Respectively in presnce of copper powder instead of using Cu(I) salts.
This reaction is called Gattermann reaction. In this reaction, Cl, Br and CN can be introduced into the benzene ring by simply treating diazonium salts with HCl, HBr, KCN. Respectively in presnce of copper powder instead of using Cu(I) salts.