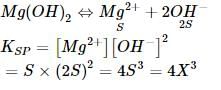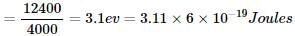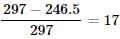AWES TGT Chemistry Mock Test - 10 - AWES TGT/PGT MCQ
30 Questions MCQ Test - AWES TGT Chemistry Mock Test - 10
Which organization has achieved Miniratna Category-I status as a Central Public Sector Enterprise (CPSE)?
Which country has the highest dengue death rate in the world?
Which country introduced a self-sovereign national digital ID for all citizens powered by blockchain technology?
Which team won the Asian Champions Trophy title in 2023, clinching their fourth championship?
The ____________ of learning in children, in which the learner perceives and responds to the whole situation, comes under Kohler's theory of understanding.
Alkaline earth metals combine with halogens to form:
A 5.2 molal aqueous solution of methyl alcohol (CH3OH) is supplied. What is the mole fraction of methyl alcohol in the solution?
2-Hexyne gives trans -2- Hexene on treatment with -
[AIEEE 2012]
In which of the following molecules would you expect the N to N bond to be shortest ?
In the following set of nucleophiles, the strongest and the weakest nucleophile respectively are
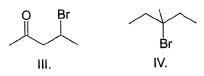
I. CH3S-
II. CH3COC-
III. HO-
IV. C6H5C-
Which of the following is true about chemisorption?
Direction (Q. Nos. 13 and 14) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
In the all oxyacids of phosphorus, each phosphorus atom is in sp3-hybridised state. All these acids contain P—OH bonds, the hydrogen atom of which are ionisable imparting acidic nature to the compound. The ‘ous’ acids (oxidation state of P is + 1 or + 3) also have P—H bonds in which hydrogens are not ionisable.
The presence of P—H bonds in these acids imparts reducing properties. The structure of some oxyacids are drawn below:
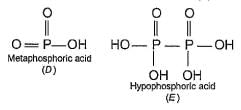
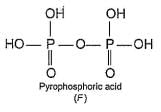
Q.
Although metaphosphoric acid is written as a monomer, it exists as a polymer (HPO3)n. The number of P—O—P bonds in cyclic trimetaphosphoric acid is
In which of the following cases, entropy of I is larger than that of II?
Which of the ligand can show linkage isomerism and acts as flexidentate ligand:
Calculate the EMF of the cell at 298 K
Pt|H2(1atm)|NaOH(xM),NaCl(xM)|AgCl(s)|Ag
If E°cl-/AgCl/Ag = + 0.222 V
Match the species in Column I with the shape in Column II.
The solubility of Mg(OH)2 is x mole/ltr. then its solubility product is -
[AIEEE-2002]
Direction (Q. Nos. 21-22) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)
A+B- (ionic) is formed in the following steps from gaseous atoms
Q.
How much energy is required to transfer one electron from A to B?
Statement Type
This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Statement I: N2 is less reactive than P4.
Statement II: Nitrogen has more electron gain enthalpy than phosphorus.
We have
I. 25 mL of 1 M NaOH
II. 10 mL of 0.50 M NaCI
On mixing the two solutions, molar concentrations of Na+, OH- and Cl- respectively, are
Which one of the following statement(s) is/are incorrect for lanthanides?
A photon of 4000 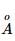 is used to break the iodine molecule, then the % of energy converted to the K.E. of iodine atoms if bond dissociation energy of I2 molecule is 246.5 kJ/mol is:
is used to break the iodine molecule, then the % of energy converted to the K.E. of iodine atoms if bond dissociation energy of I2 molecule is 246.5 kJ/mol is:
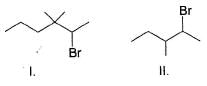
What is the increasing order of reactivity of the following in an E2 reaction with ethanolic KOH solution?
Select the correct observation about electrolysis.