NEET Exam > NEET Tests > Test: Bond Parameters - NEET MCQ
Test: Bond Parameters - NEET MCQ
Test Description
20 Questions MCQ Test - Test: Bond Parameters
Test: Bond Parameters for NEET 2025 is part of NEET preparation. The Test: Bond Parameters questions and answers have been prepared
according to the NEET exam syllabus.The Test: Bond Parameters MCQs are made for NEET 2025 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Bond Parameters below.
Solutions of Test: Bond Parameters questions in English are available as part of our course for NEET & Test: Bond Parameters solutions in
Hindi for NEET course.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free. Attempt Test: Bond Parameters | 20 questions in 20 minutes | Mock test for NEET preparation | Free important questions MCQ to study for NEET Exam | Download free PDF with solutions
Detailed Solution for Test: Bond Parameters - Question 1
Test: Bond Parameters - Question 2
The decreasing order of the repulsive interactions between various electron pairs is:
Detailed Solution for Test: Bond Parameters - Question 2
Detailed Solution for Test: Bond Parameters - Question 3
Detailed Solution for Test: Bond Parameters - Question 4
Test: Bond Parameters - Question 5
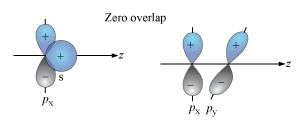
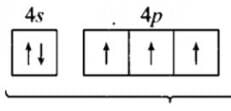
The s-orbital does not show preference to any direction because
Detailed Solution for Test: Bond Parameters - Question 5
Test: Bond Parameters - Question 6
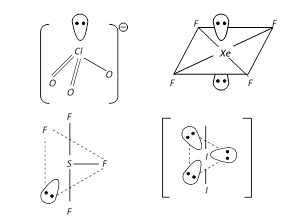
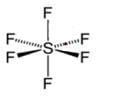
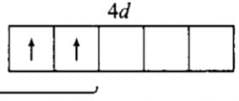
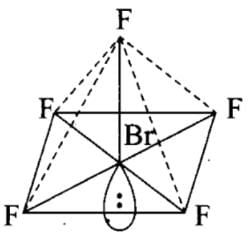
The number of lone pairs of electrons on SF4, CF4 and XeF4 are:
Detailed Solution for Test: Bond Parameters - Question 6
Test: Bond Parameters - Question 7
Pick out the pair of species having identical shapes for both the molecules.
Detailed Solution for Test: Bond Parameters - Question 7
Detailed Solution for Test: Bond Parameters - Question 8
Detailed Solution for Test: Bond Parameters - Question 9
Detailed Solution for Test: Bond Parameters - Question 10
Test: Bond Parameters - Question 11
Among the following molecules, the molecule with trigonal planar geometry is:
Detailed Solution for Test: Bond Parameters - Question 11
Detailed Solution for Test: Bond Parameters - Question 12
Detailed Solution for Test: Bond Parameters - Question 13
Test: Bond Parameters - Question 14
According to the VSEPR theory, the geometry and shape of the molecule depends upon:
Detailed Solution for Test: Bond Parameters - Question 14
Detailed Solution for Test: Bond Parameters - Question 15
Detailed Solution for Test: Bond Parameters - Question 16
Detailed Solution for Test: Bond Parameters - Question 17
Detailed Solution for Test: Bond Parameters - Question 18
Test: Bond Parameters - Question 19
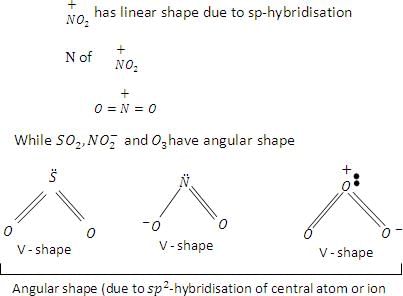
Among the following species the pair that have V-shaped geometry is:
Detailed Solution for Test: Bond Parameters - Question 19
Test: Bond Parameters - Question 20
Among the following compounds the maximum number of lone pair is present on the central atom of:
Detailed Solution for Test: Bond Parameters - Question 20
Information about Test: Bond Parameters Page
In this test you can find the Exam questions for Test: Bond Parameters solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Bond Parameters, EduRev gives you an ample number of Online tests for practice
Download as PDF




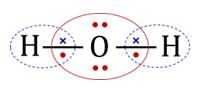
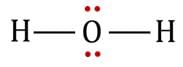
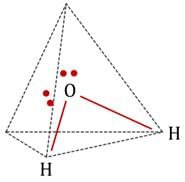
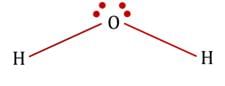
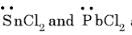 are bent shape molecule.
are bent shape molecule.