Test: Electromagnetic radiation, Bohr model - JEE MCQ
20 Questions MCQ Test - Test: Electromagnetic radiation, Bohr model
What is the angular velocity 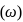 of an electron occupying second orbit of
of an electron occupying second orbit of 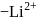 ion?
ion?
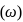 of an electron occupying second orbit of
of an electron occupying second orbit of 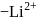 ion?
ion?The ion that is isoelectronic with CO is-
The energy of an electron in first Bohr orbit of 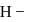 atom is
atom is 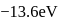 . The energy value of electron in the excited state of
. The energy value of electron in the excited state of 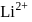 is:
is:
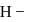 atom is
atom is 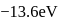 . The energy value of electron in the excited state of
. The energy value of electron in the excited state of 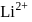 is:
is:The Bohr's energy equation for H atom reveals that the energy level of a shell is given by E = −13.58/n2eV. The smallest amount that an H atom will absorb if in ground state is
If the electron of a hydrogen atom is present in the first orbit, the total energy of the electron is
An electron, e1 is moving in the fifth stationary state, and another electron e2 is moving in the fourth stationary state. The radius of orbit of electron, e1 is five times the radius of orbit of electron, e2 calculate the ratio of velocity of electron e1(v1) to the velocity of electron e2(v2)
In a hydrogen atom, if energy of an electron 1n ground state is 13.6.ev, then that in the 2nd excited state is
What is the ratio of the atomic radius of the 5th orbit in chlorine atom and 3rd orbit in Helium atom?
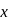 to
to 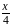 . The change in potential energy will be:
. The change in potential energy will be:
What atomic number of an element "X" would have to become so that the 4 th orbit around X would fit inside the I Bohr orbit of H atom?
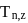 where n represents shell no. and
where n represents shell no. and 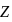 represents atomic number then the value of
represents atomic number then the value of  will be :
will be :
The energy of 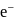 in first orbit of
in first orbit of 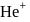 is
is 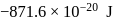 . The energy of
. The energy of 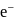 in first orbit of
in first orbit of 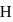 is:
is:
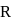 then radius of third orbit will be:
then radius of third orbit will be:
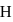 -atom is
-atom is 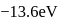 . The possible energy value of electron in the excited state of
. The possible energy value of electron in the excited state of 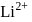 is
is
The kinetic energy of an electron in the second Bohr orbit of a hydrogen atom is [a0 is Bohr radius] :
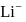 ion is represented by:
ion is represented by:
The speed of an electron in the first Bohr orbit is 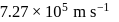 . The speed of electron in the
. The speed of electron in the 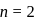 level of
level of 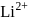 ion will be
ion will be


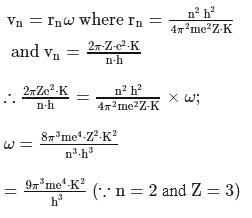

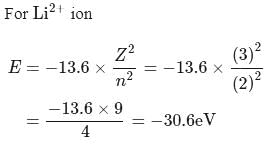
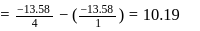
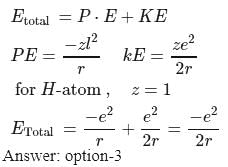
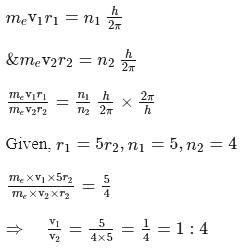
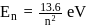 or
or 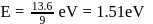
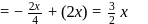
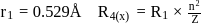
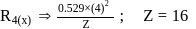
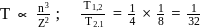

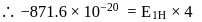
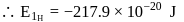
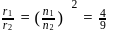
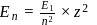
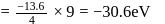
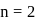 and for
and for 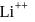 ion,
ion, 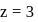
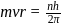
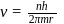
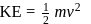
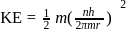
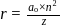
 Bohr orbit
Bohr orbit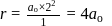
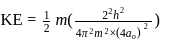
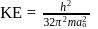
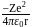
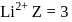 , where
, where 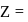 atomic number of an atom
atomic number of an atom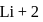 ion is
ion is 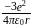
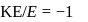
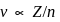 , we will have
, we will have 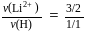
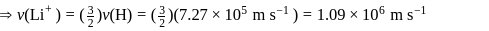
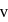 around the nucleus in a stationary orbit of radius
around the nucleus in a stationary orbit of radius 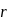 . The electrostatic force acting on the electron is balanced by the centrifugal force i.e.
. The electrostatic force acting on the electron is balanced by the centrifugal force i.e. 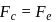
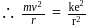 where
where 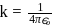
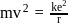 ... (1)
... (1)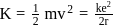 ... (2)
... (2)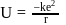
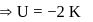 (using (2))
(using (2))










