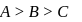Test: Periodic Trends - JEE MCQ
20 Questions MCQ Test - Test: Periodic Trends
The first ionisation energies of magnesium and aluminium are respectively given by
Electron gain enthalpy values of noble gases are positive because:
The formation of the oxide ion 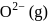 , from oxygen atom requires first an exothermic and then an endothermic step as shown below:
, from oxygen atom requires first an exothermic and then an endothermic step as shown below:
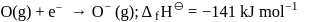
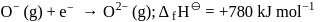
Thus process of formation of 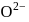 in gas phase is unfavourable even though
in gas phase is unfavourable even though 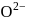 is isoelectronic with neon. It is due to the fact that
is isoelectronic with neon. It is due to the fact that
(i) Atomic radii decreases across a row of the periodic table when we move from left to right.
(ii) Atomic radii increases down the column as we move from top to bottom.
(iii) Although the order of elements is based on atomic numbers, vertical families share similar chemical properties.
Which of the statement(s) given above is/are correct?
Consider the following changes
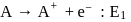 and
and 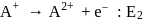
The energy required to pull out the two electrons are 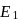 and
and 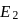 respectively. The correct relationship between two energies would be
respectively. The correct relationship between two energies would be
Ionization energies of atoms A and B are 400 and 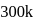 cal
cal 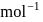 respectively. The electron affinities of these atoms are
respectively. The electron affinities of these atoms are 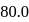 and
and 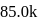 cal
cal 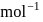 respectively. Then which is the correct statement regarding electronegativity
respectively. Then which is the correct statement regarding electronegativity 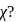
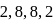 as the electronic configuration. Which one of the following statements regarding this element is not correct?
as the electronic configuration. Which one of the following statements regarding this element is not correct?
Which of the following series correctly represents relations between the elements from 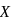 to
to 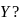
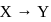
Elements X, Y and Z have atomic numbers 19,37 and 55 respectively. Which of the following statements is true about them ?
Identify the element which has highest second ionisation enthalpy.
Which property decreases from left to right across the periodic table and increases from top to bottom?
(i) Atomic radius
(ii) Electronegativity
(iii) Ionisation energy
(iv) Metallic character
N0/4 atoms of X(g) are converted into X+(g) by energy E1. N0 /4 atoms of X(g) are converted into X–(g) by energy E2. Hence, ionisation potential and electron affinity of X(g) are :
TThe formation of the oxide ion O2−(g) requires first an exothermic and then an endothermic step as shown below. 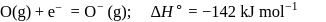
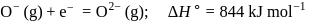
This is because
The electronic configuration of elements A,B and C are [He]2s1, [Ne]3s1 and [Ar] 4s1 respectively, which one of the following order is correct for the first ionization potentials (in kJmol−1) of A,B and C ?


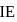 of
of 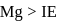 of
of 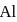
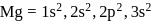
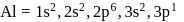
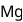 has stable configuration (fulfilled) than
has stable configuration (fulfilled) than 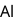 so removal of an electron is difficult hence, required more energy i.e. more ionisation energy.
so removal of an electron is difficult hence, required more energy i.e. more ionisation energy.
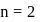 shell, also negative charge on oxygen
shell, also negative charge on oxygen 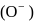 is another factor due to which incoming electron feel repulsion. Hence electron repulsion outweigh the stability gained by achieving noble gas configuration.
is another factor due to which incoming electron feel repulsion. Hence electron repulsion outweigh the stability gained by achieving noble gas configuration.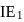 is always less than
is always less than 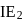 .
.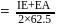
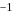 .
.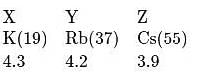
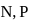 and As is
and As is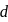 '-orbitals due to which it has higher electron affinity than nitrogen. As also has vacant
'-orbitals due to which it has higher electron affinity than nitrogen. As also has vacant 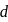 -orbitals but its atomic size is very big thus its electron affinity will be lesser than
-orbitals but its atomic size is very big thus its electron affinity will be lesser than 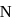 .
.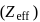 increases and so do the ionisation energy and moving down a group, effective nuclear charge
increases and so do the ionisation energy and moving down a group, effective nuclear charge 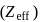 decreases and so do the ionisation energy. So, phosphorus belonging to 3 rd period will have less ionisation energy than 2nd period elements.
decreases and so do the ionisation energy. So, phosphorus belonging to 3 rd period will have less ionisation energy than 2nd period elements.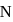 is greater than
is greater than 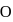 as
as 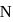 has half-filled stable electronic configuration, but after the removal of 1 electron, oxygen acquires a half-filled stable electronic configuration, the second ionisation potential of oxygen becomes more than of
has half-filled stable electronic configuration, but after the removal of 1 electron, oxygen acquires a half-filled stable electronic configuration, the second ionisation potential of oxygen becomes more than of 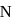 and
and 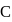 .
.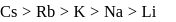
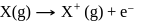

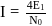
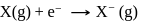
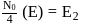
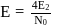
 and
and  elements decreases with the ease of removal of electron from
elements decreases with the ease of removal of electron from  to
to 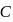 . Atomic size increase from
. Atomic size increase from 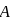 to
to 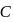 , hence ionisation potential decrease in the same order.
, hence ionisation potential decrease in the same order.