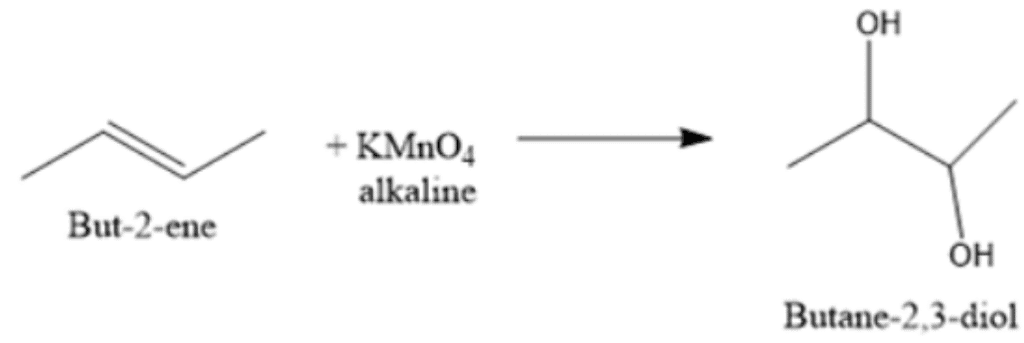
Test: Reactions of alkenes - JEE MCQ
20 Questions MCQ Test - Test: Reactions of alkenes
The major product of the following reaction sequence is
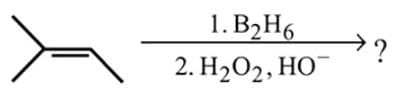
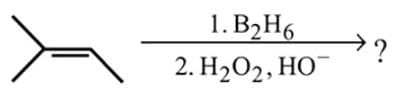
Identify the addition reaction which is not undergone by the alkenes
In the presence of peroxide, HCl and HI do not give anti-Markownikoff's addition of alkenes because:
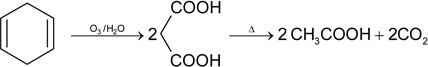
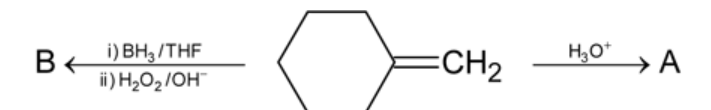
Consider the following sequence of reactions
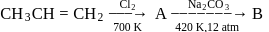
Compound 'B' is
Which one of the following compounds gives acetone (CH3)2C = O as one of the product of its ozonolysis?
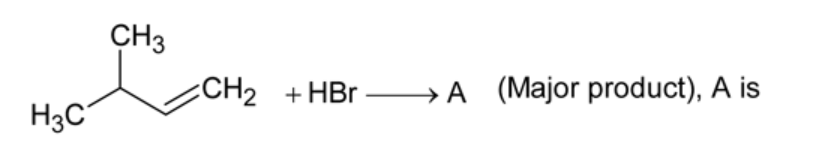
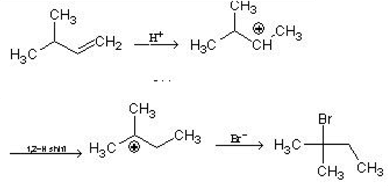
What is the major product expected from the following reaction?
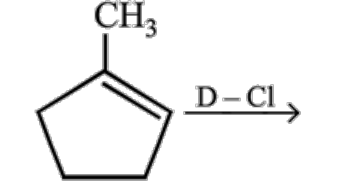
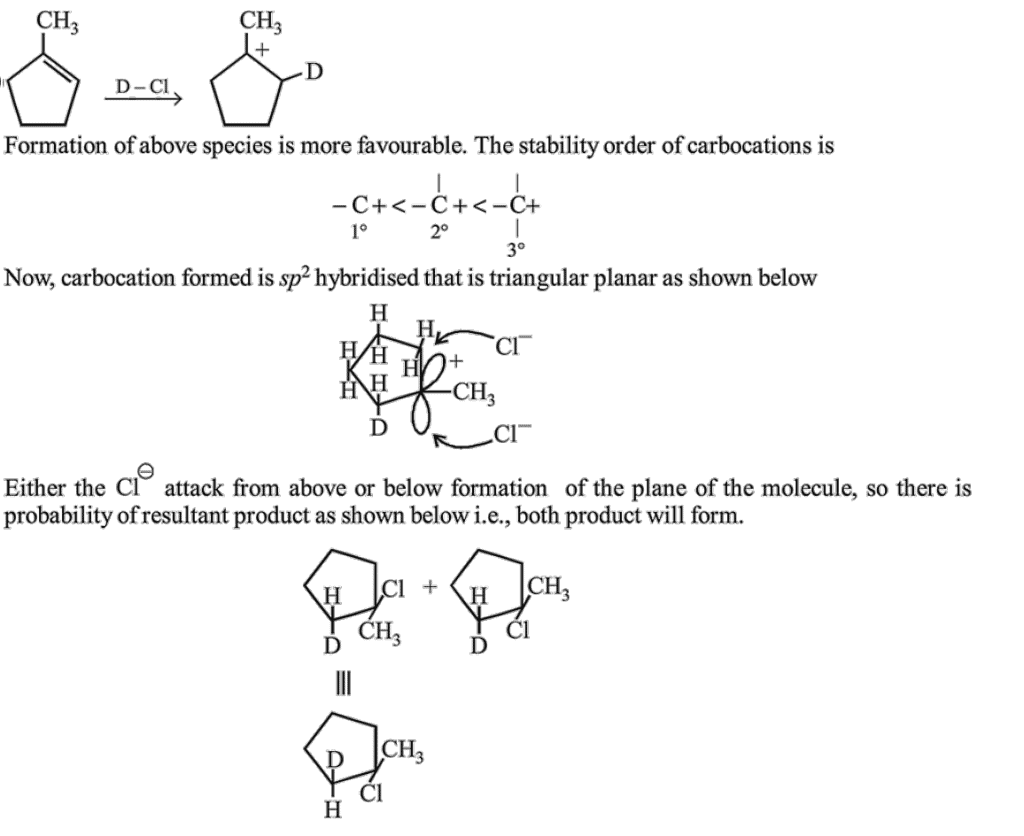
Where D is an isotope of hydrogen
Addition of HI to double bond of propene yields isopropyl iodide and not n -propyl iodide as the major product, because addition proceeds through
The principal organic product formed in the reaction,
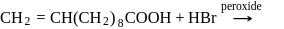 is
is
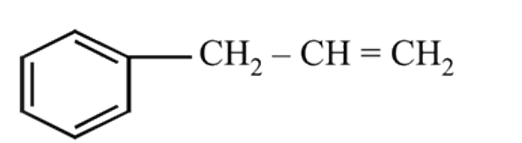
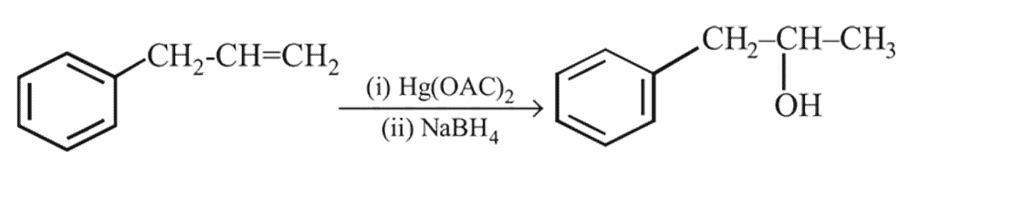
On mercuration-demercuration produces the major product:
An organic compound A(C5H8) on hydrogenation gives compound B(C5H12) ). Compound A on reductive ozonolysis give methanal and 2-ketopropanal. The compound (A) is
Hydroboration oxidation and acid hydration will not give the same products in case of
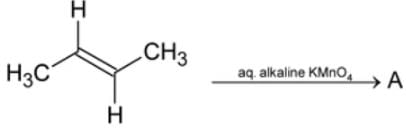
Which of the following compound does not decolourise bromine-water solution ?


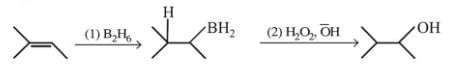

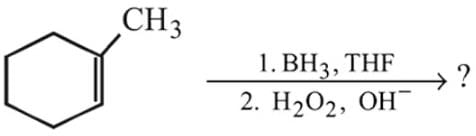
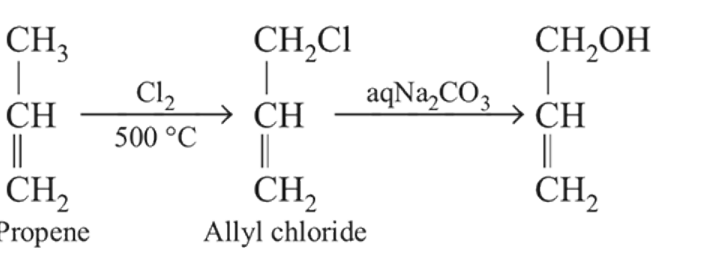
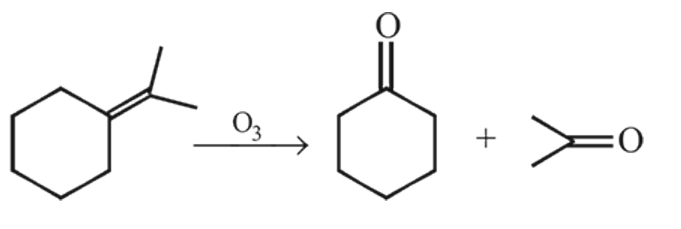
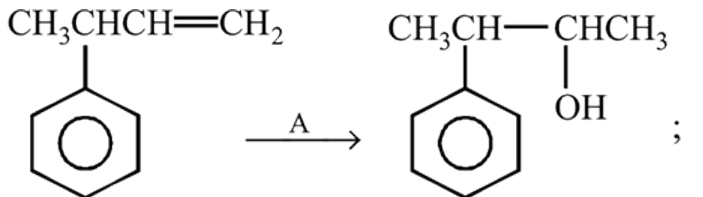
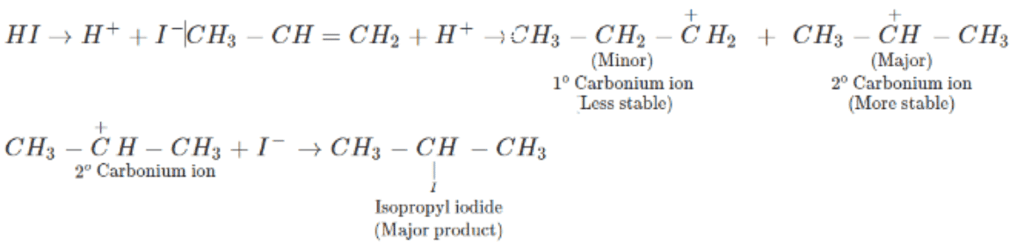
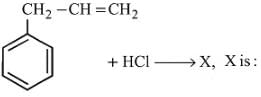
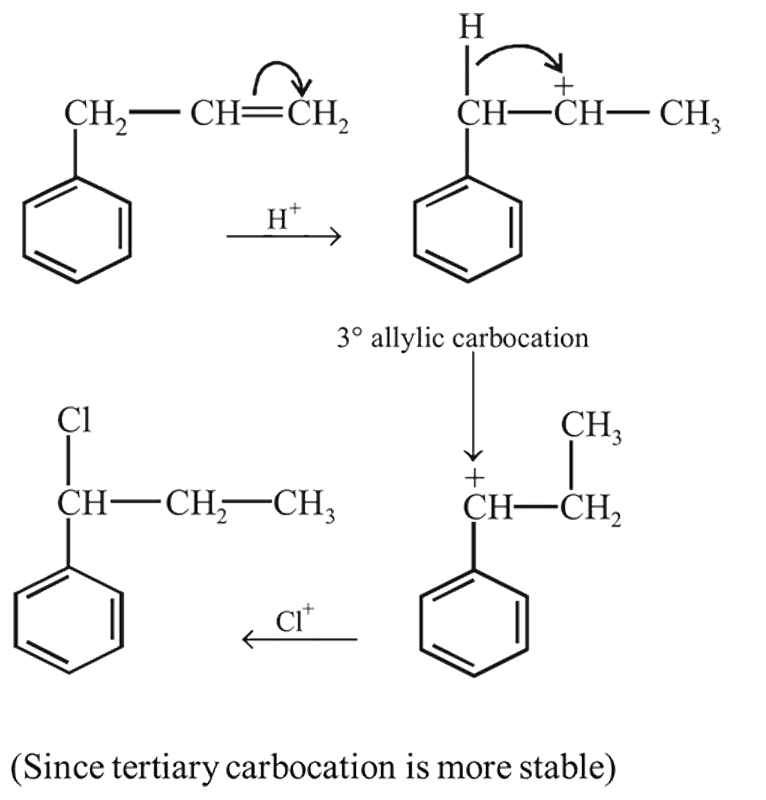
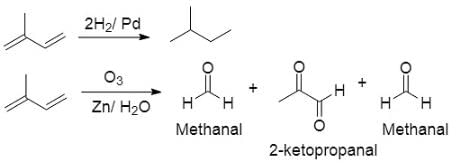
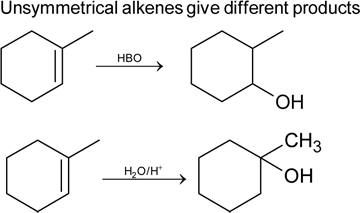
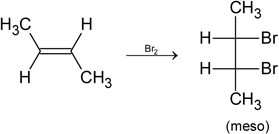
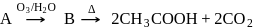 the reactant A is
the reactant A is