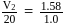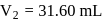Test: Raoult's and Henry's law - JEE MCQ
15 Questions MCQ Test - Test: Raoult's and Henry's law
A 5.2 molal aqueous solution of methyl alcohol (CH3OH) is supplied. What is the mole fraction of methyl alcohol in the solution?
Two 1-litre flask  and
and 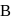 are connected to each other by a valve which is closed. Flask A has benzene in equilibrium with its vapours at
are connected to each other by a valve which is closed. Flask A has benzene in equilibrium with its vapours at 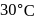 . The flask
. The flask 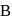 , is evacuated, and the valve is opened. Which of the following is true. If temperature is kept constant.'
, is evacuated, and the valve is opened. Which of the following is true. If temperature is kept constant.'
Vapour pressure (in torr) of an ideal solution of two liquids 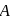 and
and 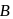 is given by :
is given by : 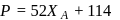 where
where 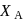 is the mole fraction of
is the mole fraction of 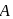 in the mixture. The vapour pressure (in torr) of equimolar mixture of the two liquids will be :
in the mixture. The vapour pressure (in torr) of equimolar mixture of the two liquids will be :
The vapour pressure (at the standard boiling point of water) of an aqueous solution containing 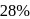 by mass of a non-volatile normal solute (molecular mass = 28) will be
by mass of a non-volatile normal solute (molecular mass = 28) will be
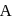 and
and 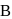 are respectively 120 and
are respectively 120 and 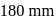 of mercury. If 2 moles of
of mercury. If 2 moles of 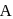 and 3 moles of
and 3 moles of 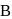 are mixed to form an ideal solution, the vapour pressure of the solution at the same temperature will be (in mm of mercury)
are mixed to form an ideal solution, the vapour pressure of the solution at the same temperature will be (in mm of mercury)
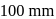 and
and 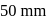 respectively. Then the mole fraction of benzene in vapour phase in contact with equimolar solution of benzene and toluene is
respectively. Then the mole fraction of benzene in vapour phase in contact with equimolar solution of benzene and toluene is
A solution at 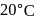 is composed of
is composed of 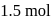 of benzene and
of benzene and 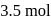 of toluene. If the vapour pressure of pure benzene and pure toluene at this temperature are
of toluene. If the vapour pressure of pure benzene and pure toluene at this temperature are 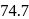 torr and
torr and 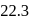 torr, respectively, then the total vapour pressure of the solution and the benzene mole fraction in equilibrium with it will be, respectively :
torr, respectively, then the total vapour pressure of the solution and the benzene mole fraction in equilibrium with it will be, respectively :
For an ideal solution of two components 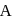 and B, which of the following is true?
and B, which of the following is true?
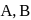 and
and 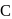 are in the order
are in the order 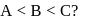
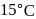 and 1 atmosphere partial pressure of hydrogen,
and 1 atmosphere partial pressure of hydrogen, 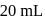 of hydrogen measured at STP dissolves in
of hydrogen measured at STP dissolves in 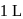 of water. If water at
of water. If water at 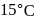 is exposed to a gaseous mixture having a total pressure of
is exposed to a gaseous mixture having a total pressure of 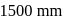 of
of 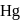 (excluding the vapour pressure of water) and containing
(excluding the vapour pressure of water) and containing 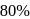 hydrogen by volumne, then the volume of hydrogen measured at STP that will dissolve in 1 L of water is
hydrogen by volumne, then the volume of hydrogen measured at STP that will dissolve in 1 L of water is
100 mL of liuqid 'A' was mixed with 25 ml of a liquid 'B' to give a non-ideal solution of A-B mixture showing (-) ve deviation from Raoult's law. The volume of this mixture would be



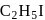 and
and 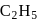 OH form non-ideal solution. Solutions that do not obey Raoult's law over the entire range of concentration are known as non-ideal solutions. Ethyl iodide and ethyl alcohol are chemically dissimilar and form a non-ideal solution.
OH form non-ideal solution. Solutions that do not obey Raoult's law over the entire range of concentration are known as non-ideal solutions. Ethyl iodide and ethyl alcohol are chemically dissimilar and form a non-ideal solution.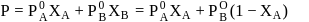
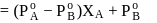
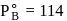 torr
torr 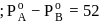
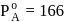 torr
torr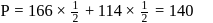 torr
torr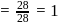 ;
;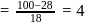
 torr
torr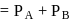
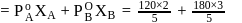
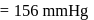
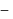 ethanol.
ethanol.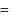 vapour pressure of pure benzene
vapour pressure of pure benzene 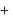 vapour pressure of toluene
vapour pressure of toluene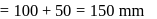
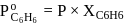
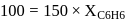
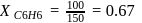
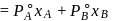
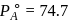 torr,
torr, 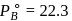 torr
torr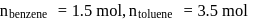
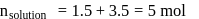

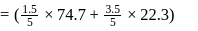 torr
torr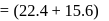 torr
torr 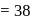 torr
torr
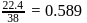
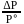 , which is equal to mole fraction of solute which is independent of temperature.
, which is equal to mole fraction of solute which is independent of temperature.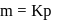 i.e. as the solubility increases, value of Henry's law constant decreases. Since
i.e. as the solubility increases, value of Henry's law constant decreases. Since 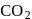 is most soluble in water among the given set of gases. Therefore
is most soluble in water among the given set of gases. Therefore 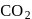 has the lowest value of Henry's law constant.
has the lowest value of Henry's law constant.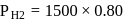
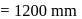 of
of 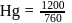 atmosphere
atmosphere 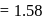
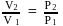 ; or
; or