Test: Determination of Rate of a Reaction, Arrhenius theory - JEE MCQ
Test Description
15 Questions MCQ Test - Test: Determination of Rate of a Reaction, Arrhenius theory
Test: Determination of Rate of a Reaction, Arrhenius theory for JEE 2025 is part of JEE preparation. The Test: Determination of Rate of a Reaction, Arrhenius theory questions and answers have been prepared
according to the JEE exam syllabus.The Test: Determination of Rate of a Reaction, Arrhenius theory MCQs are made for JEE 2025 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Determination of Rate of a Reaction, Arrhenius theory below.
Solutions of Test: Determination of Rate of a Reaction, Arrhenius theory questions in English are available as part of our course for JEE & Test: Determination of Rate of a Reaction, Arrhenius theory solutions in
Hindi for JEE course.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free. Attempt Test: Determination of Rate of a Reaction, Arrhenius theory | 15 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study for JEE Exam | Download free PDF with solutions
Test: Determination of Rate of a Reaction, Arrhenius theory - Question 1
The time taken for 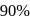 of a first order reaction to complete is approximately
of a first order reaction to complete is approximately
Detailed Solution for Test: Determination of Rate of a Reaction, Arrhenius theory - Question 1
Test: Determination of Rate of a Reaction, Arrhenius theory - Question 2
A foreign substance that increase the speed of a chemical reaction is called
Detailed Solution for Test: Determination of Rate of a Reaction, Arrhenius theory - Question 2
Test: Determination of Rate of a Reaction, Arrhenius theory - Question 3
. What will be the value of instantaneous rate of reaction from the graph?
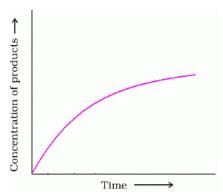

Detailed Solution for Test: Determination of Rate of a Reaction, Arrhenius theory - Question 3
Test: Determination of Rate of a Reaction, Arrhenius theory - Question 4
The rate of a chemical reaction doubles for every 10°C rise of temperature. If the temperature is raised by 50°C, the rate of the reaction increases by about
[AIEEE 2011]
Detailed Solution for Test: Determination of Rate of a Reaction, Arrhenius theory - Question 4
Test: Determination of Rate of a Reaction, Arrhenius theory - Question 5
Chemical substances speeding up rate of chemical reaction is called as
Detailed Solution for Test: Determination of Rate of a Reaction, Arrhenius theory - Question 5
Test: Determination of Rate of a Reaction, Arrhenius theory - Question 6
Which of the following reactions is not of the first order?
Detailed Solution for Test: Determination of Rate of a Reaction, Arrhenius theory - Question 6
Test: Determination of Rate of a Reaction, Arrhenius theory - Question 7
A first order reaction is 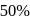 completed in 20 minutes at
completed in 20 minutes at 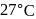 and in 5 minutes at
and in 5 minutes at 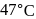 . The energy of activation of the reaction is :
. The energy of activation of the reaction is :
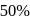 completed in 20 minutes at
completed in 20 minutes at 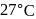 and in 5 minutes at
and in 5 minutes at 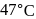 . The energy of activation of the reaction is :
. The energy of activation of the reaction is :
Detailed Solution for Test: Determination of Rate of a Reaction, Arrhenius theory - Question 7
Test: Determination of Rate of a Reaction, Arrhenius theory - Question 8
In a first-order reaction 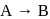 , if
, if 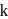 is rate constant and initial concentration of the reactant
is rate constant and initial concentration of the reactant 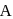 is
is 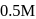 , then the halflife is
, then the halflife is
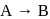 , if
, if 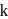 is rate constant and initial concentration of the reactant
is rate constant and initial concentration of the reactant 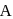 is
is 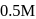 , then the halflife is
, then the halflife is
Detailed Solution for Test: Determination of Rate of a Reaction, Arrhenius theory - Question 8
Test: Determination of Rate of a Reaction, Arrhenius theory - Question 9
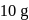 of a radioactive element is disintegrated to
of a radioactive element is disintegrated to 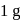 in 2.303 minutes. What is the half-life (in minutes) of that radioactive element?
in 2.303 minutes. What is the half-life (in minutes) of that radioactive element?
Detailed Solution for Test: Determination of Rate of a Reaction, Arrhenius theory - Question 9
Test: Determination of Rate of a Reaction, Arrhenius theory - Question 10
Half life period of a first-order reaction is 1386 seconds. The specific rate constant of the reaction is:
Detailed Solution for Test: Determination of Rate of a Reaction, Arrhenius theory - Question 10
Test: Determination of Rate of a Reaction, Arrhenius theory - Question 11
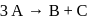 , it would be a zero order reaction when
, it would be a zero order reaction when
Detailed Solution for Test: Determination of Rate of a Reaction, Arrhenius theory - Question 11
Test: Determination of Rate of a Reaction, Arrhenius theory - Question 12
The decomposition of a substance follows first order kinetics. Its concentration is reduced to 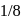 th of its initial value in 24 minutes. The rate constant of the decomposition process in
th of its initial value in 24 minutes. The rate constant of the decomposition process in 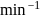 is
is
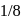 th of its initial value in 24 minutes. The rate constant of the decomposition process in
th of its initial value in 24 minutes. The rate constant of the decomposition process in 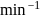 is
is
Detailed Solution for Test: Determination of Rate of a Reaction, Arrhenius theory - Question 12
Test: Determination of Rate of a Reaction, Arrhenius theory - Question 13
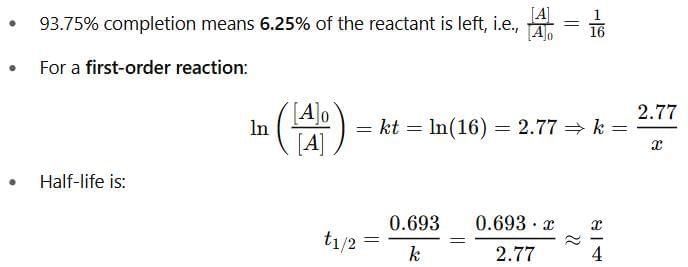
The time required for completion of 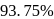 of a first order reaction is
of a first order reaction is 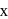 minutes. The half life of it (in minutes) is
minutes. The half life of it (in minutes) is
Detailed Solution for Test: Determination of Rate of a Reaction, Arrhenius theory - Question 13
Test: Determination of Rate of a Reaction, Arrhenius theory - Question 14
The halflife of a radioactive element is 20 min. The time interval between the stages of its 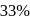 and
and 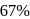 decay is
decay is
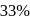 and
and 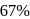 decay is
decay is
Detailed Solution for Test: Determination of Rate of a Reaction, Arrhenius theory - Question 14
Test: Determination of Rate of a Reaction, Arrhenius theory - Question 15
The reaction 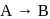 follows first order kinetics. The time taken for
follows first order kinetics. The time taken for 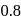 mole of
mole of 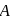 to produce
to produce 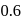 mole of
mole of 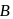 is 1 hour. What is the time taken for conversion of
is 1 hour. What is the time taken for conversion of 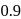 mole of
mole of 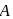 to produce
to produce 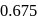 mole of B?
mole of B?
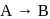 follows first order kinetics. The time taken for
follows first order kinetics. The time taken for 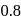 mole of
mole of 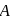 to produce
to produce 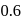 mole of
mole of 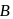 is 1 hour. What is the time taken for conversion of
is 1 hour. What is the time taken for conversion of 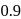 mole of
mole of 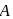 to produce
to produce 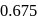 mole of B?
mole of B?
Detailed Solution for Test: Determination of Rate of a Reaction, Arrhenius theory - Question 15
Information about Test: Determination of Rate of a Reaction, Arrhenius theory Page
In this test you can find the Exam questions for Test: Determination of Rate of a Reaction, Arrhenius theory solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Determination of Rate of a Reaction, Arrhenius theory, EduRev gives you an ample number of Online tests for practice
Download as PDF


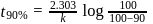 (I)
(I)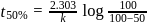 (II)
(II)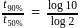
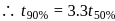

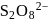 and
and  .
.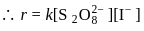
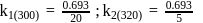
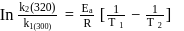
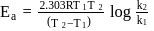
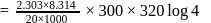
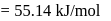
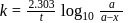
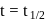
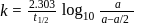
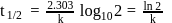
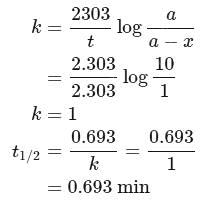
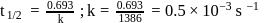
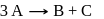
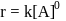 , i. e the rate remains same at any concentration of '
, i. e the rate remains same at any concentration of ' 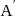 . i.e independent upon concentration of A.
. i.e independent upon concentration of A.
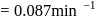
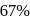 to
to 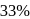 is almost half the concentration change.
is almost half the concentration change.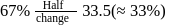
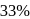 and
and 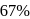 decay is same as
decay is same as 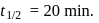
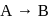 For a first order reaction
For a first order reaction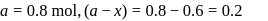
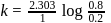 or
or 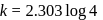
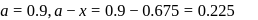
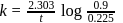
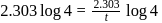
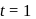 hour
hour










