Test: Preparation and Properties of colloids - JEE MCQ
Test Description
15 Questions MCQ Test - Test: Preparation and Properties of colloids
Test: Preparation and Properties of colloids for JEE 2025 is part of JEE preparation. The Test: Preparation and Properties of colloids questions and answers have been prepared
according to the JEE exam syllabus.The Test: Preparation and Properties of colloids MCQs are made for JEE 2025 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Preparation and Properties of colloids below.
Solutions of Test: Preparation and Properties of colloids questions in English are available as part of our course for JEE & Test: Preparation and Properties of colloids solutions in
Hindi for JEE course.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free. Attempt Test: Preparation and Properties of colloids | 15 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study for JEE Exam | Download free PDF with solutions
Test: Preparation and Properties of colloids - Question 1
During the action of enzyme "Zymase", glucose is converted into_____, with the liberation of carbon dioxide gas.
Detailed Solution for Test: Preparation and Properties of colloids - Question 1
Detailed Solution for Test: Preparation and Properties of colloids - Question 2
Test: Preparation and Properties of colloids - Question 3
The efficiency of an enzyme in catalysing a reaction is due to its capacity
Detailed Solution for Test: Preparation and Properties of colloids - Question 3
Test: Preparation and Properties of colloids - Question 4
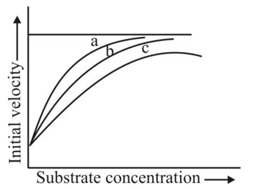
The figure given below shows three velocity-substrate concentration curves for an enzyme reaction. What do the curves 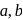 and c depict respectively?
and c depict respectively?
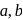 and c depict respectively?
and c depict respectively?
Detailed Solution for Test: Preparation and Properties of colloids - Question 4
Test: Preparation and Properties of colloids - Question 5
The density of gold is 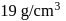 . If
. If 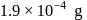 of gold is dispersed in one litre of water to give a sol having spherical gold particles of radius 10
of gold is dispersed in one litre of water to give a sol having spherical gold particles of radius 10 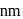 , then the number of gold particles per
, then the number of gold particles per 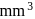 of
of
the sol will be :
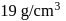 . If
. If 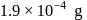 of gold is dispersed in one litre of water to give a sol having spherical gold particles of radius 10
of gold is dispersed in one litre of water to give a sol having spherical gold particles of radius 10 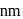 , then the number of gold particles per
, then the number of gold particles per 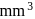 of
ofthe sol will be :
Detailed Solution for Test: Preparation and Properties of colloids - Question 5
Detailed Solution for Test: Preparation and Properties of colloids - Question 6
Test: Preparation and Properties of colloids - Question 7
Cloud or fog is a colloidal system in which the dispersed phase and the dispersion medium are
Detailed Solution for Test: Preparation and Properties of colloids - Question 7
Test: Preparation and Properties of colloids - Question 8
Which of the following constitutes irreversible colloidal system in water as dispersion medium?
Detailed Solution for Test: Preparation and Properties of colloids - Question 8
Test: Preparation and Properties of colloids - Question 9
Which one of the following statements is not correct in respect of lyophilic sols?
Detailed Solution for Test: Preparation and Properties of colloids - Question 9
Detailed Solution for Test: Preparation and Properties of colloids - Question 10
Test: Preparation and Properties of colloids - Question 11
The stabilization of the dispersed phase in a lyophobic sol is due to
Detailed Solution for Test: Preparation and Properties of colloids - Question 11
Test: Preparation and Properties of colloids - Question 12
A detergent 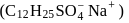 solution becomes a colloidal sol at a concentration of
solution becomes a colloidal sol at a concentration of 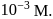 On an average
On an average 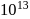 colloidal particles are present in
colloidal particles are present in 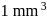 . What is the average number of ions which are contained by one colloidal particle (micelle)?
. What is the average number of ions which are contained by one colloidal particle (micelle)?  Given:
Given: 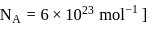
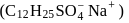 solution becomes a colloidal sol at a concentration of
solution becomes a colloidal sol at a concentration of 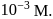 On an average
On an average 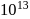 colloidal particles are present in
colloidal particles are present in 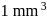 . What is the average number of ions which are contained by one colloidal particle (micelle)?
. What is the average number of ions which are contained by one colloidal particle (micelle)?  Given:
Given: 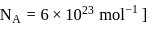
Detailed Solution for Test: Preparation and Properties of colloids - Question 12
Detailed Solution for Test: Preparation and Properties of colloids - Question 13
Test: Preparation and Properties of colloids - Question 14
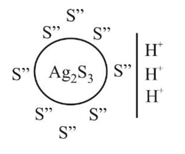
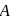 white precipitate of
white precipitate of 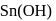 is peptized with di.
is peptized with di. 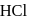 . The sol particle will carry
. The sol particle will carry
Detailed Solution for Test: Preparation and Properties of colloids - Question 14
Test: Preparation and Properties of colloids - Question 15
The sol prepared by Bredig's Arc method is X and the charge of sol particles of it is q. 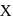 and q are respectively.
and q are respectively.
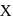 and q are respectively.
and q are respectively.
Detailed Solution for Test: Preparation and Properties of colloids - Question 15
Information about Test: Preparation and Properties of colloids Page
In this test you can find the Exam questions for Test: Preparation and Properties of colloids solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Preparation and Properties of colloids, EduRev gives you an ample number of Online tests for practice
Download as PDF


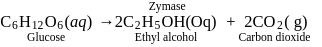

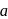 shows normal enzyme reaction, while curve
shows normal enzyme reaction, while curve 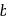 shows a competitive inhibition reaction, in which competitive inhibitors that resemble the substrate molecules, bind to the active site of the enzyme, whereas, curve
shows a competitive inhibition reaction, in which competitive inhibitors that resemble the substrate molecules, bind to the active site of the enzyme, whereas, curve 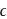 shows non-competitive inhibition reaction, in which the inhibitor binds to a part of the enzyme or enzyme substrate complex, other than the active site, known as the allosteric site.
shows non-competitive inhibition reaction, in which the inhibitor binds to a part of the enzyme or enzyme substrate complex, other than the active site, known as the allosteric site.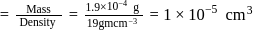
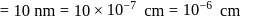
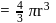
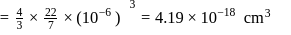
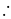 No. of gold sol particles in
No. of gold sol particles in 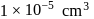
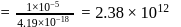
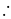 No. of gold sol particles in one
No. of gold sol particles in one 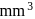
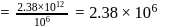
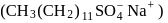 in 1 litre solution
in 1 litre solution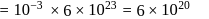
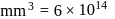
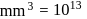 No. of molecules per colloidal particle
No. of molecules per colloidal particle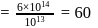
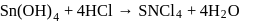
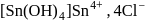 (Positive charge)
(Positive charge)















