Test: Group 17 Elements: Halogens - JEE MCQ
Test Description
15 Questions MCQ Test - Test: Group 17 Elements: Halogens
Test: Group 17 Elements: Halogens for JEE 2025 is part of JEE preparation. The Test: Group 17 Elements: Halogens questions and answers have been prepared
according to the JEE exam syllabus.The Test: Group 17 Elements: Halogens MCQs are made for JEE 2025 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Group 17 Elements: Halogens below.
Solutions of Test: Group 17 Elements: Halogens questions in English are available as part of our course for JEE & Test: Group 17 Elements: Halogens solutions in
Hindi for JEE course.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free. Attempt Test: Group 17 Elements: Halogens | 15 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study for JEE Exam | Download free PDF with solutions
Test: Group 17 Elements: Halogens - Question 1
A white precipitate is obtained on hydrolysis of
Detailed Solution for Test: Group 17 Elements: Halogens - Question 1
Test: Group 17 Elements: Halogens - Question 2
Which of the following arrangements gives the correct order of increasing oxidation number of iodine?
Detailed Solution for Test: Group 17 Elements: Halogens - Question 2
Test: Group 17 Elements: Halogens - Question 3
Mark the element which shows only one oxdation state
Detailed Solution for Test: Group 17 Elements: Halogens - Question 3
Test: Group 17 Elements: Halogens - Question 4
Which of the following is not correctly matched?
Detailed Solution for Test: Group 17 Elements: Halogens - Question 4
Detailed Solution for Test: Group 17 Elements: Halogens - Question 5
Test: Group 17 Elements: Halogens - Question 6
What are the products formed when chlorine is passed through aqueous hypo solution?
Detailed Solution for Test: Group 17 Elements: Halogens - Question 6
Test: Group 17 Elements: Halogens - Question 7
Which one of the following reactions does not occur?
Detailed Solution for Test: Group 17 Elements: Halogens - Question 7
Test: Group 17 Elements: Halogens - Question 8
Of the interhalogen 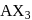 compounds,
compounds, 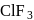 is most reactive but
is most reactive but 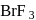 has higher conductance
has higher conductance
in liquid state. This is because
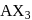 compounds,
compounds, 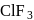 is most reactive but
is most reactive but 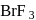 has higher conductance
has higher conductancein liquid state. This is because
Detailed Solution for Test: Group 17 Elements: Halogens - Question 8
Test: Group 17 Elements: Halogens - Question 9
Which of the following is the best description for the behaviour of bromine in the reaction given below ? 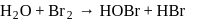
Detailed Solution for Test: Group 17 Elements: Halogens - Question 9
Detailed Solution for Test: Group 17 Elements: Halogens - Question 10
Test: Group 17 Elements: Halogens - Question 11
Which of the following is correct about the reaction?
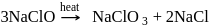
Detailed Solution for Test: Group 17 Elements: Halogens - Question 11
Detailed Solution for Test: Group 17 Elements: Halogens - Question 12
Test: Group 17 Elements: Halogens - Question 13
The correct order of reactivity of halogens with alkalies is
Detailed Solution for Test: Group 17 Elements: Halogens - Question 13
Test: Group 17 Elements: Halogens - Question 14
Among the following oxoacids, the correct order of acid strength is
Detailed Solution for Test: Group 17 Elements: Halogens - Question 14
Test: Group 17 Elements: Halogens - Question 15
Which of the following species is not a pseudo halide :-
Detailed Solution for Test: Group 17 Elements: Halogens - Question 15
Information about Test: Group 17 Elements: Halogens Page
In this test you can find the Exam questions for Test: Group 17 Elements: Halogens solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Group 17 Elements: Halogens, EduRev gives you an ample number of Online tests for practice
Download as PDF


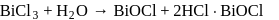

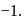
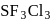 is
is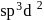 , hence octahedral
, hence octahedral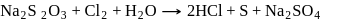
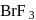 dissociates into
dissociates into 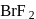 and
and 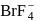 ions most easily.
ions most easily.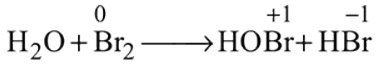
 and also decreases from 0 to
and also decreases from 0 to  . Thus it is oxidised as well as reduced.
. Thus it is oxidised as well as reduced.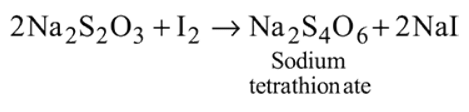
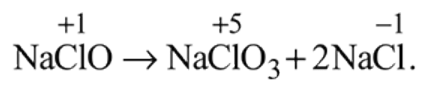
 and
and  results in
results in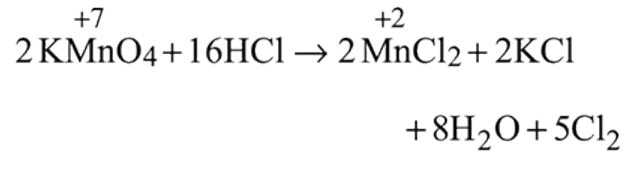
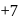 to
to 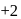 hence reduction occurs and
hence reduction occurs and 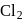 is formed.
is formed.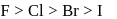
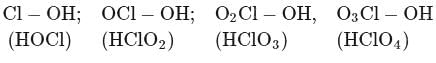
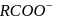 is not a pseudohlide ion. Remaining
is not a pseudohlide ion. Remaining 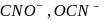 and
and 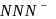 are pseudohlide ions.
are pseudohlide ions.










