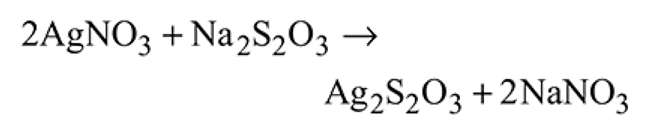Test: Compounds of Transition Metals - JEE MCQ
Test Description
20 Questions MCQ Test - Test: Compounds of Transition Metals
Test: Compounds of Transition Metals for JEE 2025 is part of JEE preparation. The Test: Compounds of Transition Metals questions and answers have been prepared
according to the JEE exam syllabus.The Test: Compounds of Transition Metals MCQs are made for JEE 2025 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Compounds of Transition Metals below.
Solutions of Test: Compounds of Transition Metals questions in English are available as part of our course for JEE & Test: Compounds of Transition Metals solutions in
Hindi for JEE course.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free. Attempt Test: Compounds of Transition Metals | 20 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study for JEE Exam | Download free PDF with solutions
Test: Compounds of Transition Metals - Question 1
In the laboratory, manganese (II) salt is oxidised to permanganate ion in aqueous solution by
Detailed Solution for Test: Compounds of Transition Metals - Question 1
Test: Compounds of Transition Metals - Question 2
When 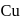 reacts with
reacts with 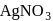 solution, the reaction takes place is
solution, the reaction takes place is
Detailed Solution for Test: Compounds of Transition Metals - Question 2
Detailed Solution for Test: Compounds of Transition Metals - Question 3
Test: Compounds of Transition Metals - Question 4
Which of the following compounds gives red precipitate with 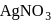 ?
?
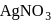 ?
?
Detailed Solution for Test: Compounds of Transition Metals - Question 4
Test: Compounds of Transition Metals - Question 5
When horn silver ore is dissolved in excess of sodium cyanide solution compound formed is
Detailed Solution for Test: Compounds of Transition Metals - Question 5
Detailed Solution for Test: Compounds of Transition Metals - Question 6
Test: Compounds of Transition Metals - Question 7
If 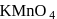 is reduced by oxalic acid in an acidic medium then oxidation number of
is reduced by oxalic acid in an acidic medium then oxidation number of 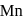 changes from
changes from
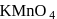 is reduced by oxalic acid in an acidic medium then oxidation number of
is reduced by oxalic acid in an acidic medium then oxidation number of 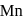 changes from
changes from
Detailed Solution for Test: Compounds of Transition Metals - Question 7
Test: Compounds of Transition Metals - Question 8
In the preparation of 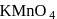 pyrolusite
pyrolusite 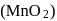 is first converted to potassium manganate
is first converted to potassium manganate 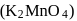 . In this conversion, the oxidation state of manganese changes from
. In this conversion, the oxidation state of manganese changes from
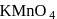 pyrolusite
pyrolusite 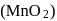 is first converted to potassium manganate
is first converted to potassium manganate 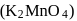 . In this conversion, the oxidation state of manganese changes from
. In this conversion, the oxidation state of manganese changes from
Detailed Solution for Test: Compounds of Transition Metals - Question 8
Test: Compounds of Transition Metals - Question 9
In an alkaline condition 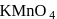 reacts as follows:
reacts as follows:
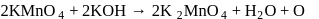
Its equivalent weight is
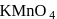 reacts as follows:
reacts as follows: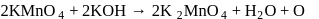
Its equivalent weight is
Detailed Solution for Test: Compounds of Transition Metals - Question 9
Test: Compounds of Transition Metals - Question 10
In which of the following compounds manganese has oxidation number equal to that of iodine in 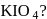
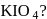
Detailed Solution for Test: Compounds of Transition Metals - Question 10
Test: Compounds of Transition Metals - Question 11
The colour of 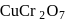 solution in water is green because
solution in water is green because
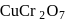 solution in water is green because
solution in water is green because
Detailed Solution for Test: Compounds of Transition Metals - Question 11
Test: Compounds of Transition Metals - Question 12
A compound of iron exists as a dimer in vapour state. It is hygroscopic in nature and dissolves in water giving brown acidic solution. The compound is
Detailed Solution for Test: Compounds of Transition Metals - Question 12
Detailed Solution for Test: Compounds of Transition Metals - Question 13
Test: Compounds of Transition Metals - Question 14
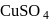 (aq.)
(aq.) 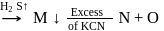
Then final products 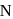 and
and 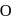 are respectively.
are respectively.
Detailed Solution for Test: Compounds of Transition Metals - Question 14
Test: Compounds of Transition Metals - Question 15
Which of the following statements is false ?
Detailed Solution for Test: Compounds of Transition Metals - Question 15
Test: Compounds of Transition Metals - Question 16
What is the change in the oxidation state of 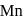 in the reaction of
in the reaction of 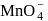 with
with 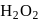 in acidic medium?
in acidic medium?
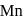 in the reaction of
in the reaction of 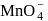 with
with 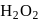 in acidic medium?
in acidic medium?
Detailed Solution for Test: Compounds of Transition Metals - Question 16
Test: Compounds of Transition Metals - Question 17
Of Cr 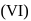 as
as 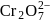 and
and 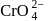 , which is better oxidising agent?
, which is better oxidising agent?
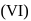 as
as 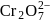 and
and 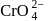 , which is better oxidising agent?
, which is better oxidising agent?
Detailed Solution for Test: Compounds of Transition Metals - Question 17
Detailed Solution for Test: Compounds of Transition Metals - Question 18
Test: Compounds of Transition Metals - Question 19
Which of the following molecules is colourless?
Detailed Solution for Test: Compounds of Transition Metals - Question 19
Test: Compounds of Transition Metals - Question 20
The black compound formed during the reaction between sodium thiosulphate and silver nitrate is
Detailed Solution for Test: Compounds of Transition Metals - Question 20
Information about Test: Compounds of Transition Metals Page
In this test you can find the Exam questions for Test: Compounds of Transition Metals solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Compounds of Transition Metals, EduRev gives you an ample number of Online tests for practice
Download as PDF


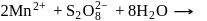
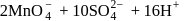
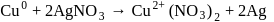
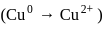

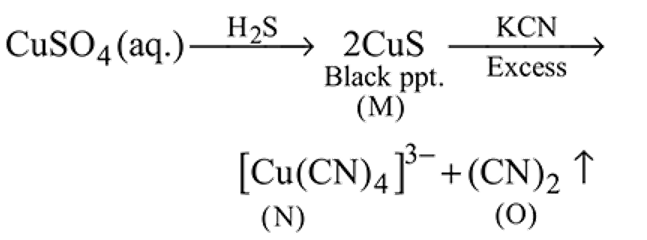
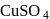 reacts with excees
reacts with excees 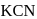 solution to form:
solution to form: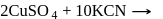
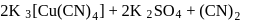
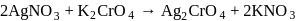
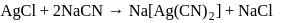
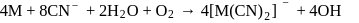
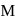 . It is
. It is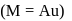 .
.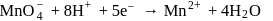
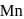 changes form
changes form 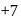 to
to 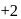 ).
).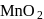 (O.S. of
(O.S. of 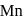 is
is 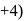 in
in 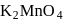 (O.S.of Mn is +6). Hence O.S. changes by 2 .
(O.S.of Mn is +6). Hence O.S. changes by 2 .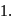 Hence eq. wt. is
Hence eq. wt. is 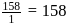
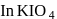 , O.S of I is
, O.S of I is 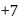 and in
and in 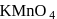 , O.S. of
, O.S. of 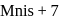
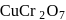 is due to blue colour of
is due to blue colour of 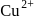 ions and yellow colour of
ions and yellow colour of 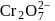 ions
ions and brown due to
and brown due to 
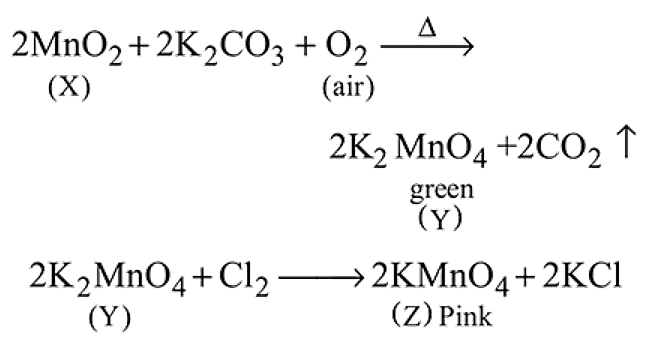
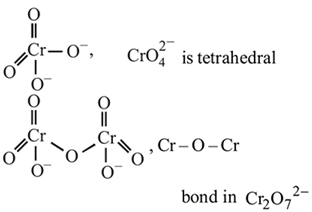
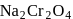 is a secondary standard solution in volumetry as it is hygroscopic in nature.
is a secondary standard solution in volumetry as it is hygroscopic in nature. is greater than
is greater than  because of smaller size of
because of smaller size of 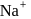 . So,
. So, 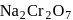 is more soluble than
is more soluble than 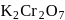
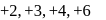 and +7 .
and +7 .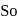 , in acidic medium on reaction of
, in acidic medium on reaction of 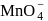 with
with 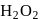 it changes from 7 to 2 .
it changes from 7 to 2 .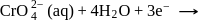
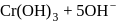 ,
,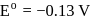
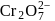 (aq.)
(aq.) 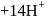 (aq.)
(aq.) 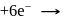
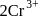 (aq.)
(aq.) 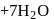 ,
,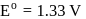
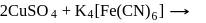
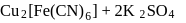
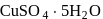 is blue in colour. The ligand (water) molecules causes splitting of
is blue in colour. The ligand (water) molecules causes splitting of 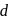 -orbitals. This facilitated
-orbitals. This facilitated 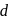 -
- 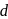 transition and colour.
transition and colour.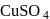 is colourless. In the absence of ligand (water) molecules, splitting of
is colourless. In the absence of ligand (water) molecules, splitting of 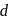 -orbitals is not possible. Hence,
-orbitals is not possible. Hence, 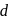 -
- 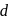 transition is not possible. Hence, option (b) is correct.
transition is not possible. Hence, option (b) is correct.