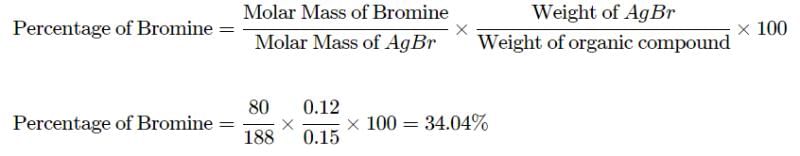Test: Analysis of Organic Compounds - NEET MCQ
10 Questions MCQ Test - Test: Analysis of Organic Compounds
Detection of nitrogen, sulphur, halogens and phosphorus in an organic compound is done by:
In case both nitrogen and sulphur is present in an organic compound with sodium fusion extract a blood red colour is formed that is of:
When a compound is heated with copper(ii) oxide it is the test for?
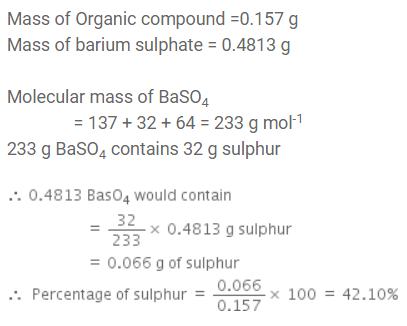
In sulphur estimation, 0.157 g of an organic compound gave 0.4813 g of barium sulphate. The percentage of sulphur in the compound is:
A compound when treated with sodium peroxide and the solution is boiled with nitric acid and then treated with ammonium molybdate is the test for:
The white precipitate which is formed in the detection of chloride ion is due to the formation of:
In Carius method of estimation of halogen, 0.15 g of an organic compound gave 0.12 g of AgBr. The percentage of bromine in the compound is:
A pale yellow precipitate soluble in ammonium hydroxide solution indicates the presence of:
In the test of nitrogen the Prussian blue color formed is of: