Test: Crystalline & Amorphous Solids (Old NCERT) - JEE MCQ
25 Questions MCQ Test - Test: Crystalline & Amorphous Solids (Old NCERT)
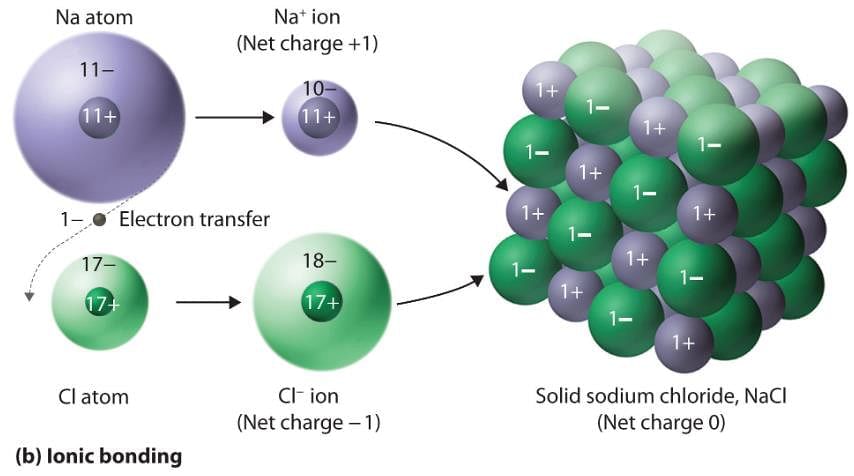
In which of the following solids, ions of opposite charges are held together by strong electrostatic forces of attraction?
Which of the following crystalline solids have highest melting point?
Amorphous solids are also known as:
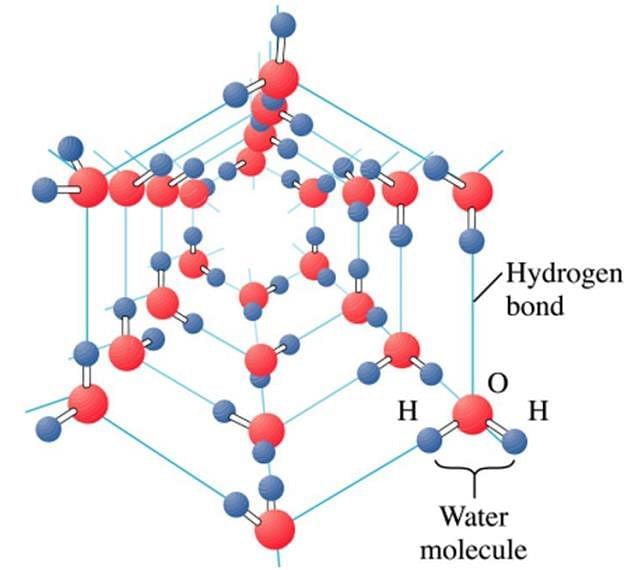
What are the basic particles of ice crystals?
Why some of the physical properties of solids show different values when measured along different directions in the same crystals?
Which of the following properties is generally exhibited by amorphous solids?
Which of the following is NOT a molecular solid?
Hydrogen bonding occurs in which type of crystalline solids?
Which of the following is an amorphous solid?
Why ancient glass becomes milky?
Which is not a characteristic of crystalline solids?
Which type of solids are held by weak dispersion forces?
Silicon is found in nature in the forms of ________.
Which state of matter is incompressible?
Why do metallic solids conducts electricity?
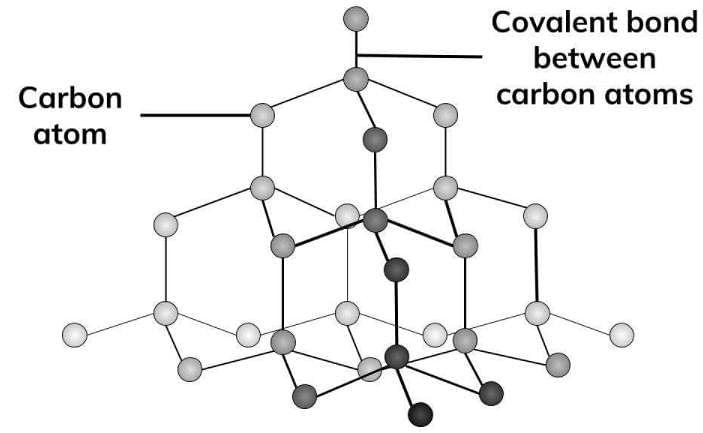
What are the basic constituent particles forming diamond crystals?
A friend in your chemistry class is struggling to understand why crystalline solids are grouped into four main types: network, molecular, ionic, and metallic. Which explanation below will BEST help him begin to understand why chemists might have these groups?
Which of the following is a non-conductor in solid state but good conductor in molten state?
Which of the following properties is NOT exhibited by metallic solids?
Among the following solids, which one shows the strongest bonding?
Why ice is a crystalline compound?
Which of the following is a covalent crystal?
The points which shows the position of atoms in a crystal are called as _________.