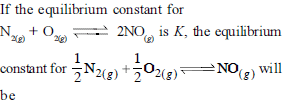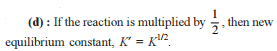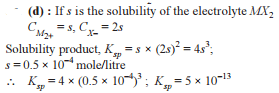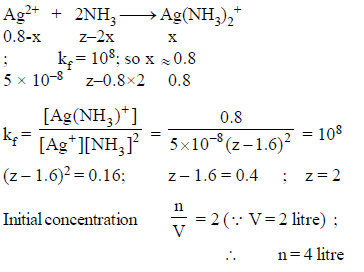Chemical Equilibrium - 1 - JEE MCQ
30 Questions MCQ Test - Chemical Equilibrium - 1
Direction (Q. Nos. 1-9) This section contains 9 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. For the reaction in equilibrium,
I. CO(g) + 1/2O2(g) 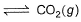
II. 2CO(g) + O2 (g) 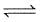 2CO2 (g)
2CO2 (g)

Then
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
I. CO(g) + 1/2O2(g)
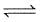 2CO2 (g)
2CO2 (g)Then
For the following equilibrium at 298 K,
CO(g) + H2O (g) 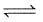 CO2(g) + H2(g);
CO2(g) + H2(g);
Δ f G° (in kcal mol-1) of CO = - 32.81, CO2 = - 94.26, H2O = - 54.64, H2 = 0.0 then, degree of dissociation of CO(g) is
CO(g) + H2O (g)
 CO2(g) + H2(g);
CO2(g) + H2(g);Δ f G° (in kcal mol-1) of CO = - 32.81, CO2 = - 94.26, H2O = - 54.64, H2 = 0.0 then, degree of dissociation of CO(g) is
Direction (Q. Nos. 10) This sectionis based on statement I and Statement II. Select the correct answer from the code given below.
Q. Statement I
For a reaction 2NO2(g)  N2O4 (g) variation of (log10 K) with (T-1) is represented as
N2O4 (g) variation of (log10 K) with (T-1) is represented as
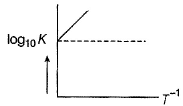
Statement II
Association o f NO2 to N2O4 is an exotherm ic efiange.
 N2O4 (g) variation of (log10 K) with (T-1) is represented as
N2O4 (g) variation of (log10 K) with (T-1) is represented asDirection (Q. Nos. 12-13) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)
1 mole H2(g) and 0.2 mole CO2(g) are introduced in a vacuum flask at 450°C and 0.5 atm.
H2(g) + CO2 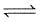 H2O(G) + CO(g)
H2O(G) + CO(g)
Analysis shows that mixture contains 10 moles per cent steam. Also equilibrium constant increases by one per cent per degree around 450°C.(log 1.1 = 0.0414)
Q. Equilibrium constant Kp is
Direction (Q. No. 14) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
The hydrogenation of pyridine (C5H5N) to piperidine (C5H11N)
C5H5N(g) + 3H2(g) 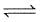 C5H11N(g)
C5H11N(g)
is an equilibrium process whose equilibrium constant (Kp)is given by
Match the thermodynamics parameters in column I with their respective values in column II.
How many moles NH3 must be added to 2.0 litre of 0.80 M AgNO3 in order to reduce the Ag+ concentration to 5 × 10–8 M. Kf of [Ag(NH3)2
+] = 108