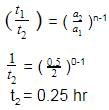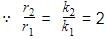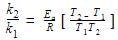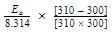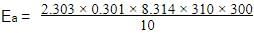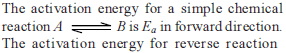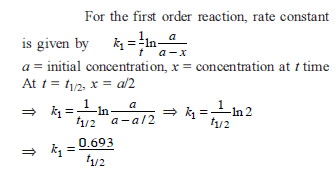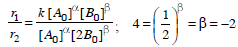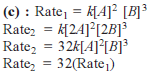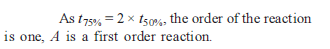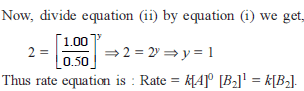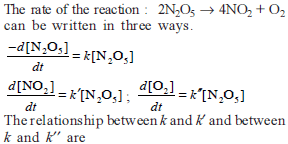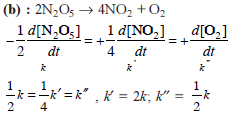Chemical Kinetics - 1 - JEE MCQ
30 Questions MCQ Test - Chemical Kinetics - 1
A chemical reaction [2A] + [2B] + [C] → product follows the rate equation : 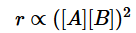 then order of reaction is - [AIEEE-2002]
then order of reaction is - [AIEEE-2002]
For the reaction system: 2NO(g) + O2(g) → 2NO2(g) volume is suddenly reduced to half its value by increasing the pressure on it. If the reaction is of first order with respect to O2 and second order with repect to NO, the rate of reaction will –
[AIEEE-2003]
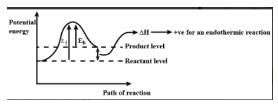
Consider an endothermic reaction X → Y with the activation energies Eb and Ef for the backward and forward reactions, respectively. In general
[AIEEE-2005]
Rate of reaction can be expressed by Arrhenius equation as k = Ae–E/RT , In this equation, E represents
[AIEEE 2006]
The following mechanism has been proposed for the reaction of NO with Br2 to form NOBr :
NO(g) + Br2 (g) NOBr2 (g)
NOBr2 (g) + NO (g) → 2 NOBr (g)
If the second step is the rate determining step, the order of the reaction with respect to NO (g) is -
[AIEEE 2006]
The time for half life period of a certain reaction A → products is 1 hour. When the initial concentration of the reactant ‘A’, is 2.0 mol L–1, how much time does it take for its concentration to come from 0.50 to 0.25 mol L–1 if it is a zero order reaction ?
[AIEEE 2010]
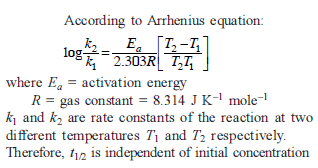
The rate of a reaction doubles when its temperature changes from 300 K to 310 K. Activation energy of such a reaction will be: (R = 8.314 JK–1 mol–1 and log 2 = 0.301)
[IIT Mains 2013]
For the reaction 2A + B → C, the values of initial rate at different reactant concentrations are given in the table below. The rate law for the reaction is :