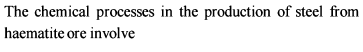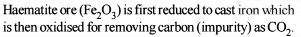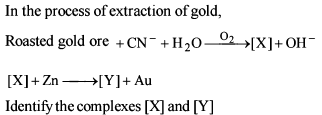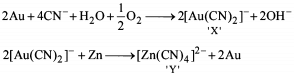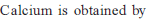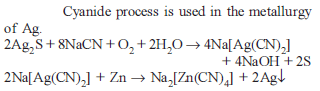General Principles and Processes of Isolation of Metals - 1 - JEE MCQ
Test Description
12 Questions MCQ Test - General Principles and Processes of Isolation of Metals - 1
General Principles and Processes of Isolation of Metals - 1 for JEE 2025 is part of JEE preparation. The General Principles and Processes of Isolation of Metals - 1 questions and answers have been prepared
according to the JEE exam syllabus.The General Principles and Processes of Isolation of Metals - 1 MCQs are made for JEE 2025 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for General Principles and Processes of Isolation of Metals - 1 below.
Solutions of General Principles and Processes of Isolation of Metals - 1 questions in English are available as part of our course for JEE & General Principles and Processes of Isolation of Metals - 1 solutions in
Hindi for JEE course.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free. Attempt General Principles and Processes of Isolation of Metals - 1 | 12 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study for JEE Exam | Download free PDF with solutions
Detailed Solution for General Principles and Processes of Isolation of Metals - 1 - Question 1
General Principles and Processes of Isolation of Metals - 1 - Question 2
Main source of lead is PbS. It is converted to Pb by :
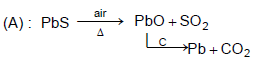
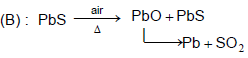
Self - reduction pocess is :
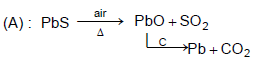
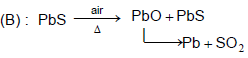
Detailed Solution for General Principles and Processes of Isolation of Metals - 1 - Question 2
General Principles and Processes of Isolation of Metals - 1 - Question 3
Among the following statements, the incorrect one is :
Detailed Solution for General Principles and Processes of Isolation of Metals - 1 - Question 3
General Principles and Processes of Isolation of Metals - 1 - Question 4
Which of the following statement is incorrect about the extractive metallurgy of copper ?
Detailed Solution for General Principles and Processes of Isolation of Metals - 1 - Question 4
Detailed Solution for General Principles and Processes of Isolation of Metals - 1 - Question 5
Detailed Solution for General Principles and Processes of Isolation of Metals - 1 - Question 6
Detailed Solution for General Principles and Processes of Isolation of Metals - 1 - Question 7
General Principles and Processes of Isolation of Metals - 1 - Question 8
Which of the following process is used in the extractive metallurgy of magnesium ?
Detailed Solution for General Principles and Processes of Isolation of Metals - 1 - Question 8
General Principles and Processes of Isolation of Metals - 1 - Question 9
NaCN is sometimes added in the froth flotation process as a depressant when ZnS and PbS minerals are expected because :
Detailed Solution for General Principles and Processes of Isolation of Metals - 1 - Question 9
General Principles and Processes of Isolation of Metals - 1 - Question 10
Match the column (I) and (II) and select the correct answer using the codes given below.
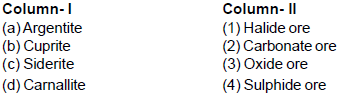
Detailed Solution for General Principles and Processes of Isolation of Metals - 1 - Question 10
Detailed Solution for General Principles and Processes of Isolation of Metals - 1 - Question 11
General Principles and Processes of Isolation of Metals - 1 - Question 12
The one that is not a carbonate ore is :
Detailed Solution for General Principles and Processes of Isolation of Metals - 1 - Question 12
Information about General Principles and Processes of Isolation of Metals - 1 Page
In this test you can find the Exam questions for General Principles and Processes of Isolation of Metals - 1 solved & explained in the simplest way possible.
Besides giving Questions and answers for General Principles and Processes of Isolation of Metals - 1, EduRev gives you an ample number of Online tests for practice
Download as PDF