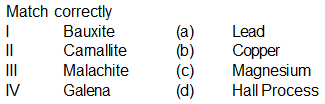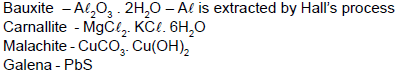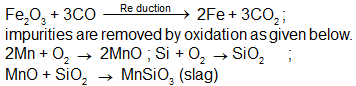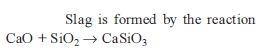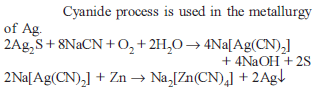General Principles and Processes of Isolation of Metals - 2 - JEE MCQ
30 Questions MCQ Test - General Principles and Processes of Isolation of Metals - 2
Assertion : For the extraction of iron, haematite ore is used.
Reason : Haematite is a carbonate ore fo iron.
Match column (I) with column (II) and select the correct answer using codes given below in the lists.

The chemical processes in the production of steel from haematite ore involve :
Which does not represent correct method ?
Match List-I with List-II and select the correct answer using the codes given below the lists.

Main function of frothers is:
With respect to an ore, Ellingham diagram helps to predict the feasibility of its -
Among the following statements, the incorrect one is :
Main function of frothers is:
Which does not represent correct method ?
The one that is not a carbonate ore is :
NaCN is sometimes added in the froth flotation process as a depressant when ZnS and PbS minerals are expected because :
The chemical composition of “slag“ formed during the smelting process in the extraction of copper is :
Which of the following statements is correct regarding the slag obtained during the extraction of a metal like copper or iron ?
When haematite ore is burnt in air with coke along with lime at 200°C, the process not only produces steel but also produces an important compound (A), which is useful in making building materials. The compound (A) is