Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) - JEE MCQ
Test Description
10 Questions MCQ Test - Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep)
Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) for JEE 2025 is part of JEE preparation. The Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) questions and answers have been prepared
according to the JEE exam syllabus.The Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) MCQs are made for JEE 2025 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) below.
Solutions of Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) questions in English are available as part of our course for JEE & Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) solutions in
Hindi for JEE course.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free. Attempt Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) | 10 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study for JEE Exam | Download free PDF with solutions
Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) - Question 1
How many degrees of freedom do non linear triatomic gas molecules has?
Detailed Solution for Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) - Question 1
Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) - Question 2
If a gas has n degree of freedom, ratio of principal specific heats of the gas is
Detailed Solution for Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) - Question 2
Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) - Question 3
O2 is a __________ molecule , and has __________ translational degrees of freedom
Detailed Solution for Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) - Question 3
Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) - Question 4
What is law of equipartition of energy?
Detailed Solution for Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) - Question 4
Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) - Question 5
The degree of freedom for diatomic gas is:
Detailed Solution for Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) - Question 5
Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) - Question 6
1calorie = ?
Detailed Solution for Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) - Question 6
Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) - Question 7
The degree of freedom for tri atomic gas is:
Detailed Solution for Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) - Question 7
Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) - Question 8
On which of the following factors, does the average kinetic energy of gas molecules depend?
Detailed Solution for Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) - Question 8
Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) - Question 9
The average distance a molecule can travel without colliding is called
Detailed Solution for Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) - Question 9
Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) - Question 10
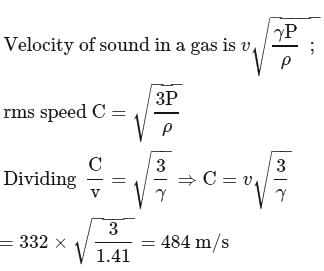
The velocity of sound in air is 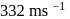 at NTP. Find the rms speed of air molecules at NTP.
at NTP. Find the rms speed of air molecules at NTP. 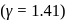
Detailed Solution for Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) - Question 10
Information about Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) Page
In this test you can find the Exam questions for Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep) solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Kinetic theory-Degree of Freedom & Kinetic Theory of Gases(26 Sep), EduRev gives you an ample number of Online tests for practice
Download as PDF