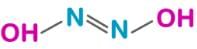
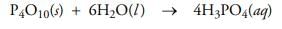Test: Main Group- 3 - Chemistry MCQ
30 Questions MCQ Test - Test: Main Group- 3
If hydrogen sulphide gas is passed through an acidified solution containing a mixture of the sulphates of cadmium, nickel and zinc, which sulphide /sulphides will be precipitated:
Consider the coordination compound, K2[Cu(CN)4]. A coordinate covalent bond exists between
Reaction of Cu metal with conc. HNO3 will produce Cu(NO3)2 and:
Among the three allotropes of an element, one is a good electrical conductor, the second one is known to be one of the hardest materials and the third one has a molecule form with an icosahedral structure. The element is:
Which of the following interhalogen compounds does not exist:
Based on Wade’s rules of electron counting, structure of carborane, CB8H14, is expected to be:
XeF6 cannot be handled in a quartz vessel, because it reacts with SiO2 forming:
Metallic potassium on treatment with liquid NH3 in the presence of catalytic amount of Fe2O3 provides:
What are A, B and C in the following reactions:
Here A, B and C respectively are:
Which one of the following contain (3c–2e) bonds:
(I) Mg(CH3)2
(II) BeCl2
(III) BeH2
(IV) Be(NO3)2
Which ones of the following compounds do not exist:
(I) AuXe4
(II) BeCl2
(III) ArF2
(IV) He–F
Among the molecules, BiF3, BiCl3 BiBr3 and BiI3, the one which is most coloured is:
Given the following reaction conditions for the formations of the fluorides of Xe:
In the preparation of P4O6, a mixture of N2 and oxygen is used instead of pure oxygen. The reason is:
Arsenic, antimony and bismuth react with concentrated HNO3. These give respect ively:
One mole of magnesium nitride on the reaction with an excess of water gives
Solution of alkali metals in liquid NH3 conducts electricity. It is due to formation of: